New intermediate for preparing heparin pentasaccharide and preparation method thereof
一种肝素五糖、中间体的技术,应用在糖衍生物制备、化学仪器和方法、氨基糖等方向,能够解决中间体稳定性差、终产物纯化困难、反应选择性低等问题,达到简化操作、简化后处理过程、效率高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
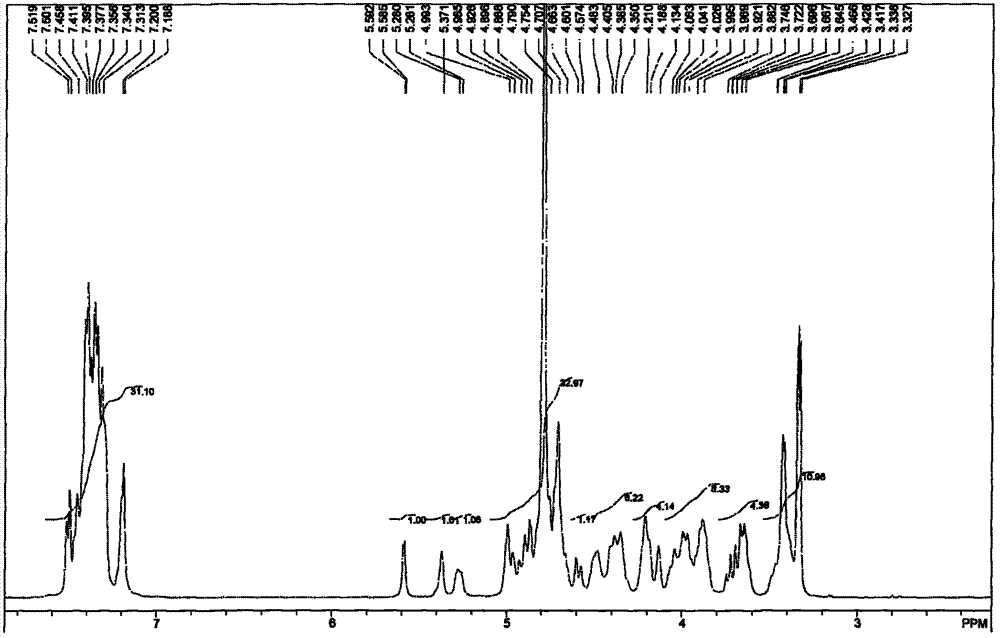
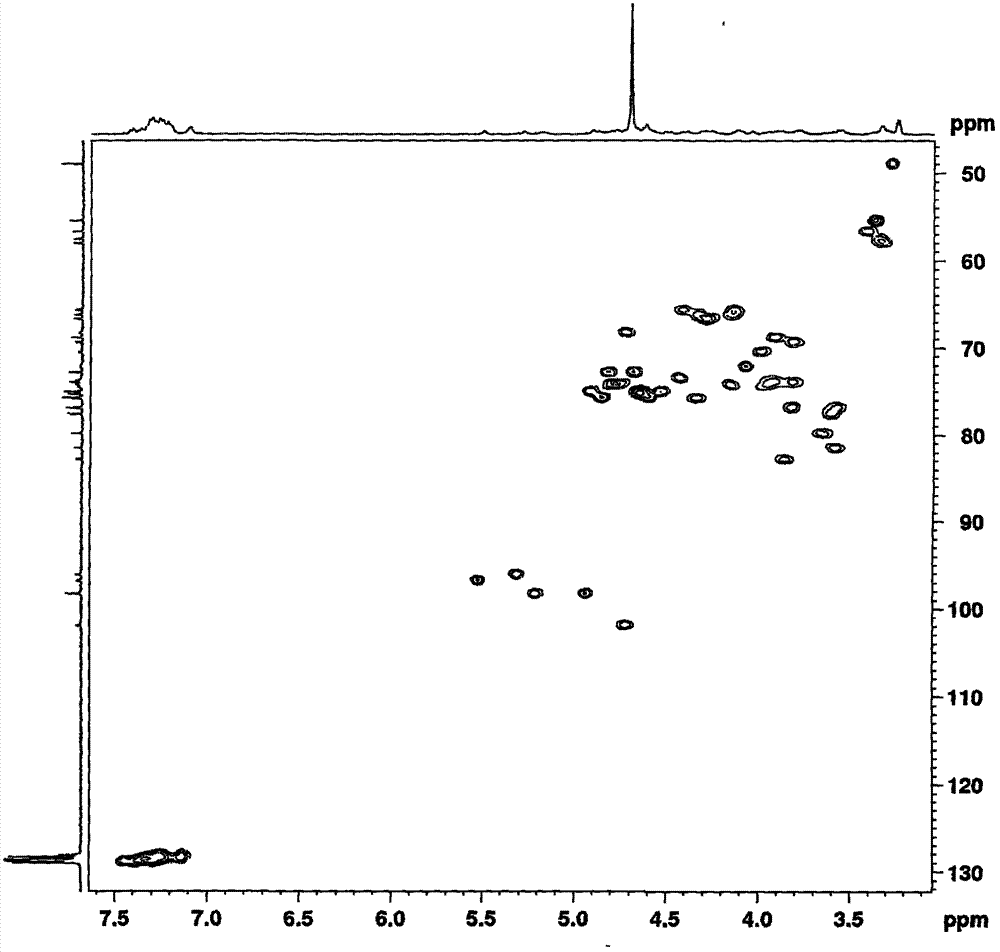
[0063] Example 1: Methyl O-(2-azido-3,4-di-O-benzyl-2-deoxy-α-D-glucopyranose)-(1→4)-O-(2, 3-Di-O-benzyl-β-D-glucopyranose)-(1→4)-O-(2-azido-2-deoxy-α-D-glucopyranose)-(1 →4)-O-(3-O-benzyl-α-L-pyraniduronic acid)-(1→4)-2-azido-3-O-benzyl-2-deoxy- Preparation of α-D-glucopyranoside (IV-1)
[0064]
[0065] The fully protected pentasaccharide III-1 compound (10g, 5.4mmol) was dissolved in tetrahydrofuran (220mL), and aqueous sodium hydroxide solution (110mL, 1.0M, 20eq, 110mmol) was added dropwise, and after the addition was completed, it was stirred overnight at room temperature until the reaction completely. Neutralize to neutral with 1M hydrochloric acid, add ethyl acetate to extract, combine the organic phases, wash the organic phases with water, 10% citric acid, and saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure to remove the solvent to obtain a foamy solid Compound IV-1 8.0 g.
[0066] ESMS: m / z=1486[M+1] + , 1484[M-1] - . ...
Embodiment 2
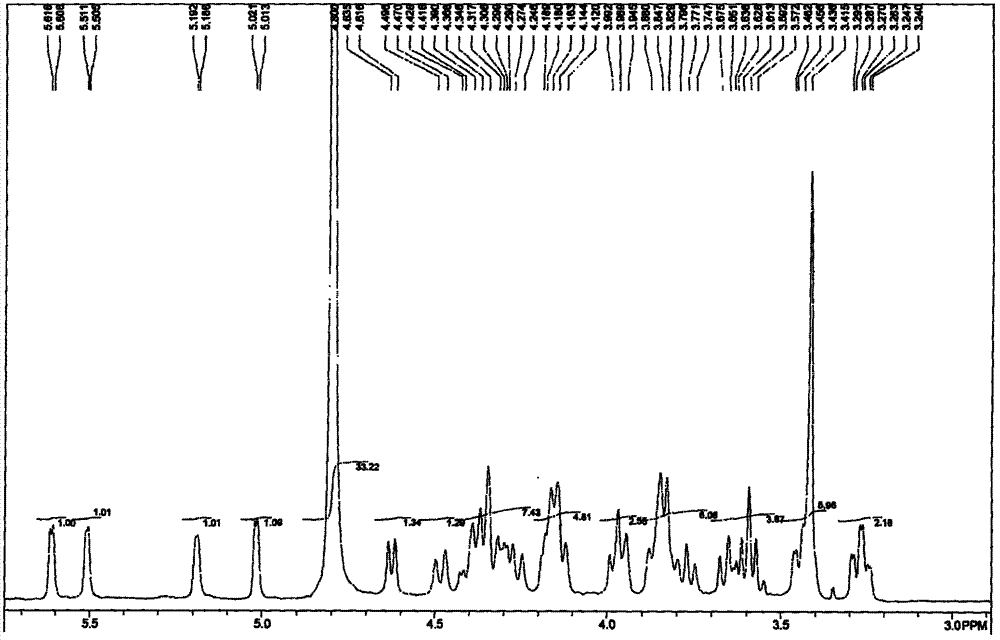
[0067] Example 2: Methyl O-(2-amino-3,4-di-O-benzyl-2-deoxy-α-D-glucopyranose)-(1→4)-O-(2,3- Di-O-benzyl-β-D-glucopyranose)-(1→4)-O-(2-amino-2-deoxy-α-D-glucopyranose)-(1→4)- O-(3-O-Benzyl-α-L-pyraniduronic acid)-(1→4)-2-amino-3-O-benzyl-2-deoxy-α-D-pyran Preparation of glucoside (I-1)
[0068]
[0069] Under the protection of nitrogen, dissolve pentasaccharide IV-1 compound (8.0g) in tetrahydrofuran (400mL), add aqueous sodium hydroxide solution (32mL, 1.0M), and drop trimethylphosphine in tetrahydrofuran solution (54mL , 1M), then slowly warmed up to room temperature, stirred overnight, until the reaction was complete, was added dropwise with dilute hydrochloric acid to neutralize to a pH of about 7, concentrated under reduced pressure to remove the solvent to obtain 8.8 g of compound I-1 as a foamy solid. The crude product was directly put into the next reaction without further purification.
[0070] HPLC purity: 90%.
[0071] ESMS: m / z 1408[M+1] + , 1406[M-1] - . ...
Embodiment 3
[0072] Example 3: Methyl O-(2-amino-3,4-di-O-benzyl-2-deoxy-α-D-glucopyranose)-(1→4)-O-(2,3- Di-O-benzyl-β-D-glucopyranose)-(1→4)-O-(2-amino-2-deoxy-α-D-glucopyranose)-(1→4)- O-(3-O-Benzyl-α-L-pyraniduronic acid)-(1→4)-2-amino-3-O-benzyl-2-deoxy-α-D-pyran Preparation of glucoside (I-1)
[0073]
[0074] Under nitrogen protection, dissolve pentasaccharide IV-1 compound (4.0g) in tetrahydrofuran (200mL), add aqueous sodium hydroxide solution (16mL, 1.0M), under ice-water bath, add triphenylphosphine (7.1g), and then Slowly raise the temperature to room temperature, stir overnight until the reaction is complete, add dropwise dilute hydrochloric acid to neutralize to a pH of about 7, and concentrate under reduced pressure to remove the solvent to obtain 11.4 g of crude compound I-1 as a foamy solid. The crude product was directly put into the next reaction without further purification.
[0075] ESMS: m / z 1408[M+1] + , 1406[M-1] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com