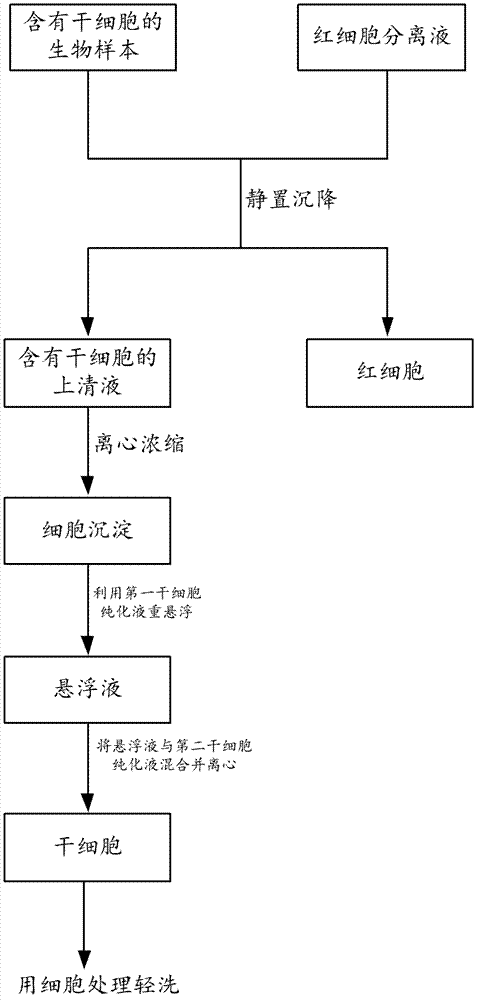
Method and kit for separating stem cells
A stem cell and cell technology, applied in the field of biomedicine, can solve the problems of high cost, high technical personnel requirements, and impure stem cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 (General Materials and General Methods)
[0035] Reagent test kit:
[0036] Reagent A: Erythrocyte Separation Solution
[0037] 0.5-0.6% methylcellulose (MC) (Switzerland FLUN K company).
[0038] The average viscosity of methylcellulose is 4,000cP. First, prepare a 0.4-0.6% by weight methylcellulose solution with pre-cooled 4°C normal saline, place it at 4°C overnight, and then shake it repeatedly until the methylcellulose is completely dissolved . The obtained methylcellulose solution was autoclaved at 0.1 MPa for 15 minutes, cooled to 4° C. and shaken vigorously. Methylcellulose becomes colorless and transparent after being completely dissolved, pH 7-8, and stored at 4°C for later use.
[0039] Reagent B: Stem Cell Purification Solution I
[0040] HBSS solution (purchased from Invitrogen Company) or GBSS solution or MEM culture fluid
[0041] Reagent C: Stem Cell Purification Solution II
[0042] 8-15% by weight iohexol solution: a predetermined amou...
Embodiment 2
[0055] Reagent A: Erythrocyte Separation Solution
[0056] 20 ng / ml human basic fibroblast growth factor (hFGF2) and 20 ng / ml human stem cell growth factor-α (pro-stem cell growth factor) were added to 0.5% methylcellulose.
[0057] Reagent B: Stem Cell Purification Solution I
[0058] 20 ng / ml human basic fibroblast growth factor (hFGF2) and 20 ng / ml human stem cell growth factor-α (original stem cell growth factor) were added to HBSS solution (purchased from Invitrogen).
[0059] Reagent C: Stem Cell Purification Solution II
[0060] 13% Nycodenz solution: Nycodenz 13g, HBSS solution (invitrogen company) 100ml, stirring > 1 hour, add 20ng / ml human basic fibroblast growth factor (hFGF2) and 20ng / ml human stem cell growth factor-α (original stem cell growth factor ).
[0061] Reagent D: cell treatment solution
[0062] Add 20ng / ml human basic fibroblast growth factor (hFGF2) and 20ng / ml human stem cell growth factor-α (original stem cell growth factor) to the phosphate buf...
Embodiment 3
[0065] Reagent A: Erythrocyte Separation Solution
[0066] 20 ng / ml human basic fibroblast growth factor (hFGF2) and 20 ng / ml human stem cell growth factor-α (pro-stem cell growth factor) were added to 0.5% methylcellulose.
[0067] Reagent B: Stem Cell Purification Solution I
[0068] 20 ng / ml human basic fibroblast growth factor (hFGF2) and 20 ng / ml human stem cell growth factor-α (original stem cell growth factor) were added to HBSS solution (purchased from Invitrogen).
[0069] Reagent C: Stem Cell Purification Solution II
[0070] 13% Nycodenz solution: Nycodenz 13g, HBSS solution (invitrogen company) 100ml, stirring > 1 hour, add 20ng / ml human basic fibroblast growth factor (hFGF2) and 20ng / ml human stem cell growth factor-α (original stem cell growth factor ).
[0071] Reagent D: cell treatment solution
[0072] Add 20ng / ml human basic fibroblast growth factor (hFGF2) and 20ng / ml human stem cell growth factor-α (original stem cell growth factor) to normal saline.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com