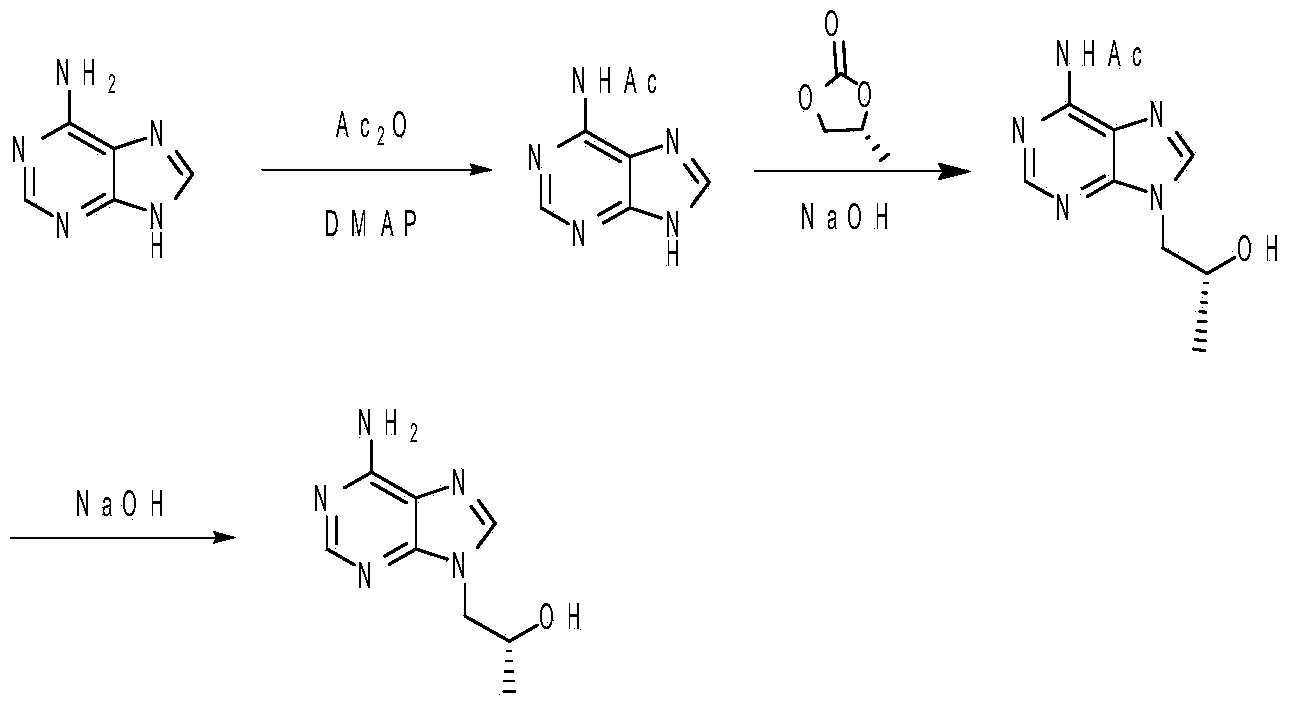
Method for synthesizing (R)-9-(2-hydroxy propyl) adenine
A technology of hydroxypropyl and adenine, which is applied in the field of medicinal chemistry, can solve the problems of affecting the yield, increasing the difficulty of industrial operation, and restricting the development of drugs, so as to achieve the effects of reducing production costs, increasing product yield, and increasing selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 250mL three-neck flask, add adenine (13.5g, 0.10mol), then add 40mL N,N-dimethylformamide, add pyridine (9.5g, 0.12mol) and 4-dimethylaminopyridine (1.2g, 0.01mol), stirring and lowering the temperature to 5°C, adding acetic anhydride (11.2g, 0.11mol) dropwise, raising the temperature to 85°C and stirring for 6 hours. Cool down to room temperature, adjust the pH to 10 with sodium carbonate, then add propylene carbonate (12.2 g, 0.12 mol), raise the temperature to 130°C and stir for reaction for 4 hours. Cool down to 70°C, add 80 mL of toluene dropwise with stirring, then slowly lower to room temperature with stirring, keep stirring for 2 hours, filter with suction, wash with 10 mL of toluene, and dry to obtain (R)-6-acetyl-9-(2- Hydroxypropyl) adenine 22.0g, yield 93.8%, high performance liquid chromatography (HPLC) purity 98.6%.
[0025] In a 100mL three-necked flask, (R)-6-acetyl-9-(2-hydroxypropyl)adenine (23.5g, 0.10mol) was added to 60mL of 10% sodium hydroxi...
Embodiment 2
[0027] In a 250mL three-neck flask, add adenine (13.5g, 0.10mol), then add 30mL N,N-dimethylformamide, 10mL N-methylpyrrolidone, add triethylamine (12.1g, 0.12mol) and 4 -Dimethylaminopyridine (1.2g, 0.01mol), cooled down to 0°C with stirring, added dropwise acetic anhydride (11.2g, 0.11mol), heated to 80-85°C and stirred for 6 hours after dropping. Cool down to room temperature, adjust the pH to 10 with sodium carbonate, then add propylene carbonate (12.2 g, 0.12 mol), raise the temperature to 135°C and stir for 3 hours. Cool down to 75°C, add 80mL of toluene dropwise under heat preservation and stirring, then slowly lower to room temperature under stirring, stir for 2 hours, filter with suction, wash with 10mL of toluene, and dry to obtain (R)-6-acetyl-9-(2- Hydroxypropyl) adenine 22.0g, yield 93.8%, HPLC purity 98.9%.
[0028] In a 100mL three-neck flask, add (R)-6-acetyl-9-(2-hydroxypropyl)adenine (23.5g, 0.10mol), add 60mL of 10% sodium hydroxide aqueous solution, and he...
Embodiment 3
[0030] In a 250mL three-neck flask, add adenine (13.5g, 0.10mol), then add 20mL N,N-dimethylformamide, 20mL N-methylpyrrolidone, add triethylamine (12.1g, 0.12mol) and 4 -Dimethylaminopyridine (1.2g, 0.01mol), cooled down to 0°C with stirring, added dropwise acetic anhydride (12.2g, 0.12mol), heated to 80-85°C and stirred for 6 hours after dropping. Cool down to room temperature, adjust the pH to 9 with sodium carbonate, then add propylene carbonate (12.2 g, 0.12 mol), raise the temperature to 130°C and stir for reaction for 4 hours. Cool down to 80°C, add 80mL of toluene dropwise under heat preservation and stirring, then slowly lower to room temperature under stirring, stir for 2 hours, filter with suction, wash with 10mL of toluene, and dry to obtain (R)-6-acetyl-9-(2- Hydroxypropyl) adenine 21.2g, yield 90.3%, HPLC purity 98.2%.
[0031] In a 100mL three-neck flask, add (R)-6-acetyl-9-(2-hydroxypropyl)adenine (23.5g, 0.10mol), add 60mL of 10% sodium hydroxide aqueous solu...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap