Method for continuously synthesizing 4-aminodiphenylamine
A technology of aminodiphenylamine and nitrodiphenylamine, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds, etc., can solve the problems of low conversion rate of raw materials and low yield of products, and achieve inhibition of initial activity, Effect of low acid content and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
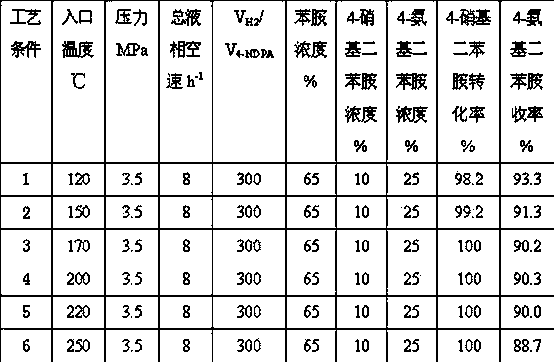
[0020] Weigh Ni / Al 2 o 3 30 milliliters of the catalyst were reduced for 16 hours at a temperature of 450° C. and a hydrogen flow rate of 1500 mL / min. At a hydrogen pressure of 3.5MPa, an inlet temperature of 150°C, and a total space velocity of the raw material liquid phase of 8 h -1 , Under the condition of hydrogen / oil volume ratio of 200:1, feed the raw material for experiment. The weight concentration of aniline in the raw material liquid is 65%, the concentration range of 4-nitrodiphenylamine is 5%-15%, and the concentration range of 4-aminodiphenylamine is 20-30%. The hydrogenation results are shown in Table 1 (the reaction time is 96 hours).
[0021] Table 1
[0022]
Embodiment 2
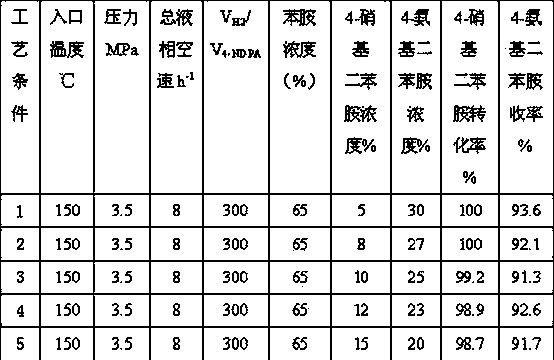
[0024] The same operation steps as in Example 1 are used to carry out reduction treatment on the catalyst. Under the conditions of hydrogen pressure of 3.5MPa, inlet temperature of 150°C, and hydrogen / oil volume ratio of 300:1, raw materials were fed for testing. The mass concentration of aniline in the raw material liquid is 65%, the concentration range of 4-nitrodiphenylamine is 10%, and the concentration range of 4-aminodiphenylamine is 25%. Liquid phase total space velocity range 6-16 h -1 , hydrogenation results are shown in Table 2 (reaction time is 96 hours).
[0025]
Embodiment 3
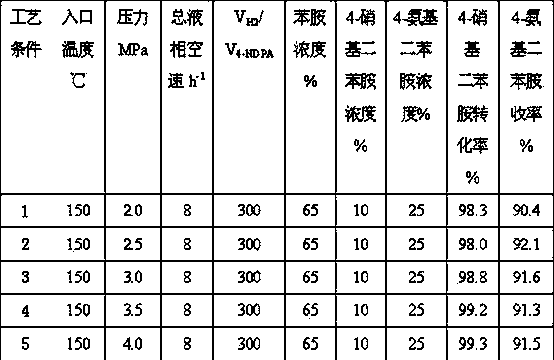
[0027] The same operation steps as in Example 1 are used to carry out reduction treatment on the catalyst. The inlet temperature is set to 150°C, and the total space velocity of the raw material liquid phase is 8 h -1 , Hydrogen / oil volume ratio 200:1. The system pressure range is 2.0-4.0 MPa. The weight concentration of aniline in the raw material liquid is 65%, the concentration range of 4-nitrodiphenylamine is 10%, and the concentration range of 4-aminodiphenylamine is 25%. The hydrogenation results are shown in Table 3 (the reaction time is 96 hours).
[0028]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com