2- methacroyloxyethyl phosphorylcholine synthesizing method
A technology of methacryloyloxyethylphosphorylcholine and hydroxyethyl methacrylate is applied in the field of synthesis of 2-methacryloyloxyethylphosphorylcholine, and can solve the problem of low yield, The problem of long route, etc., to achieve the effect of high product yield, simple and feasible process, simple and novel synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
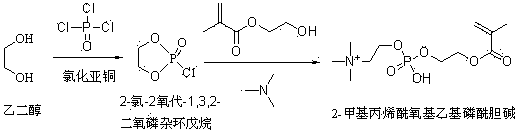
[0020] Step a, synthesis of 2-chloro-2 oxo-1,3,2-dioxaphospholane
[0021] Take 161 g (1.05 moles) of phosphorus oxychloride and 100 ml of benzene in a dry three-necked flask, o 0.5 g of cuprous chloride was added at 0°C, and then 62 g (1 mole) of ethylene glycol was added dropwise, keeping the temperature at 5 o Below C, after the dropwise addition, at 5 o Stir at below C for 1 hour, then react at 40°C for 0.5 hour, and rectify the reaction liquid to obtain 2-chloro-2-oxo-1,3,2-dioxaphospholane product, the yield is 95%, and the product purity is Up to 99%.
[0022]
[0023] Step b, synthesis of 2-methacryloyloxyethyl phosphorylcholine
[0024] Take 26 g of hydroxyethyl methacrylate (0.2 mol), 35.5 g of trimethylamine (0.6 mol), 100 mL of tetrahydrofuran, and 100 mL of acetonitrile in a dry pressure reaction flask, at -20 o 28.5 g of 2-chloro-2-oxo-1,3,2-dioxaphospholane (0.2 mol) was slowly added dropwise at ℃, and after the dropwise addition was completed, ...
Embodiment 2
[0027] Step a, synthesis of 2-chloro-2 oxo-1,3,2-dioxaphospholane
[0028] Take 62 g (1 mole) of ethylene glycol and 100 ml of benzene in a dry three-necked flask, o 0.5 g of cuprous chloride was added at 0°C, and then 161 g (1.05 moles) of phosphorus oxychloride was added dropwise, keeping the temperature at 5 o Below C, after the dropwise addition, at 5 o Stir at below C for 1 hour, then react at 40°C for 0.5 hour, and rectify the reaction solution to obtain 2-chloro-2oxo-1,3,2-dioxaphospholane product with a yield of 93% and product purity Up to 99%.
[0029]
[0030] Step b, synthesis of 2-methacryloyloxyethyl phosphorylcholine
[0031] Take 130 g of hydroxyethyl methacrylate (1 mole), 177.5 g of trimethylamine (3 moles), 500 mL of tetrahydrofuran, and 500 mL of acetonitrile in a dry pressure reaction flask, at -20 o 142.5 g of 2-chloro-2-oxo-1,3,2-dioxaphospholane (1 mole) was slowly added dropwise at ℃, and after the dropwise addition was completed, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com