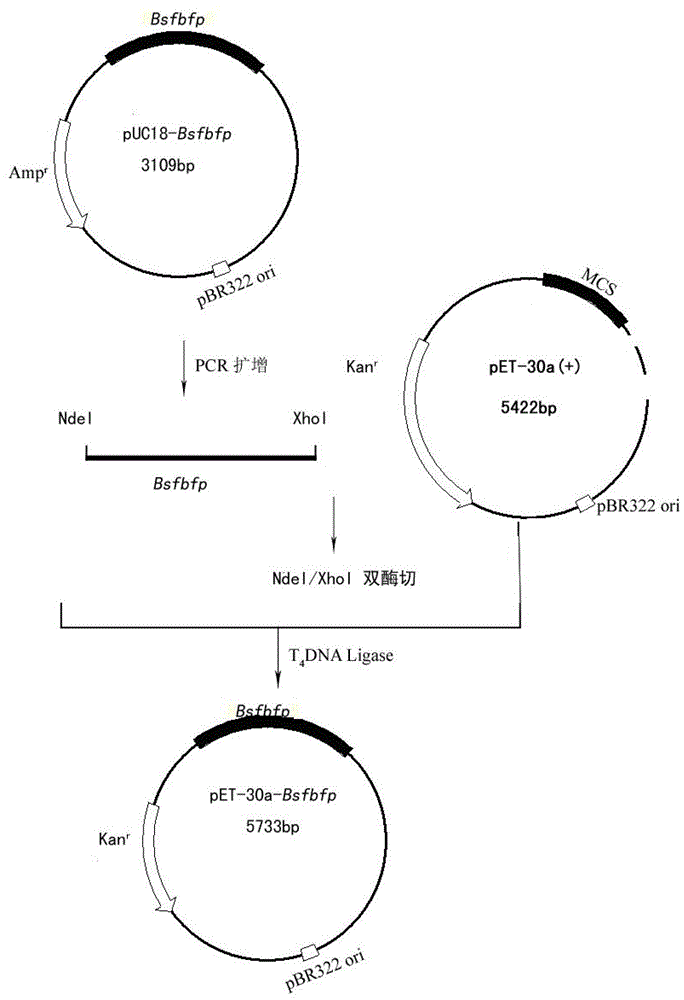
Flavin mononucleotide based fluorescence protein having modified performances
A technology of flavin mononucleotide and protein, applied in the field of flavin mononucleotide combined with fluorescent protein, to achieve the effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Selection of rationally designed mutants:
[0022] The present invention adopts foldX (Guerois, R.; Nielsen, J.E.; Serrano, L.J.Mol.Biol.2002) by starting from the LOV subunit crystal structure (pdb: 2PR5) of Bacillus subtilis flavin mononucleotide combined with blue fluorescent protein YtVA , 320, 369.) software for stable single mutant screening. The steps are briefly described as follows: First, use the 'RepairPDB' command in foldX to minimize the energy of the subunit structure, and then use the 'PositionScan' command to perform 19 kinds of natural amino acid mutations except wild protein amino acids at each position of the optimized structure, And to calculate the impact of mutations on thermal stability, 712 mutations of 94 sites in the above-identified sites showed improved thermal stability. Then, based on the fact that there are multiple mutations at a certain site, several relatively stable sites are selected; at the same time, the mutated amino acid involves...
Embodiment 2
[0070] According to the single mutations obtained above, double mutation combinations were performed, including N107F_N124F, N107Y_N124Y, and N107Y_V120I. These combined mutations were again subjected to the fluorescence detection and Tm fitting process of the above steps, and then the influence of the combined mutants on the thermal stability Tm value was obtained as shown in the table 2.
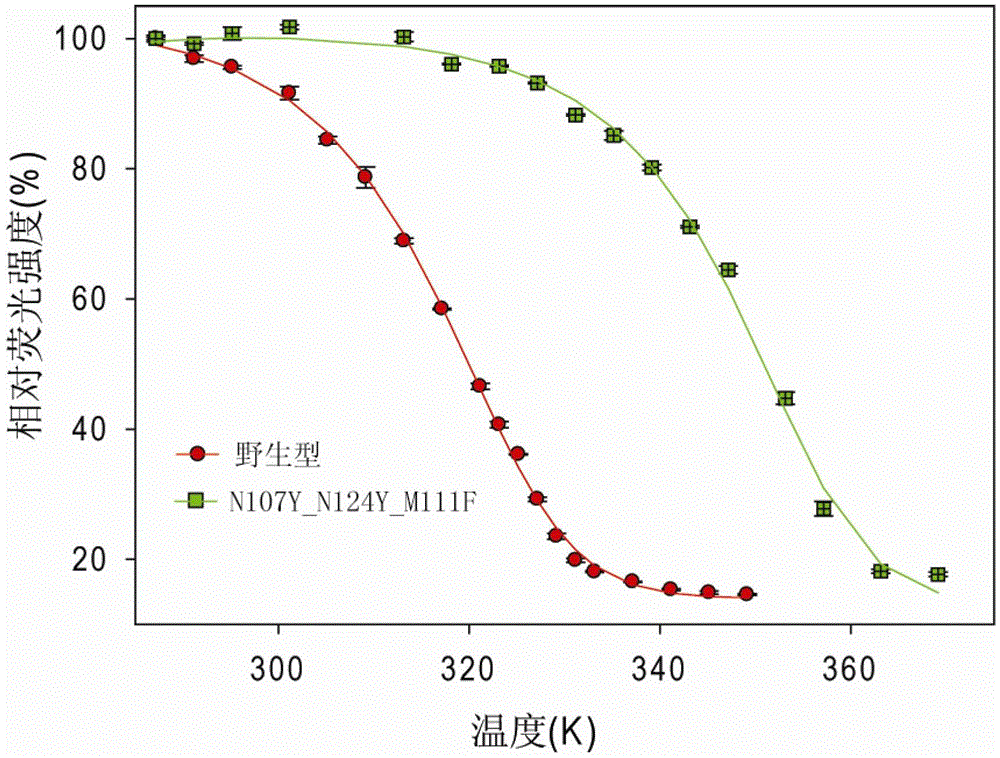
[0071] Among them, N107F_N124F and N107Y_N124Y showed higher stability than any single mutation in the double mutation, while the Tm value of N107Y_V120I was comparable to that of N107Y, indicating that this combination had no additive effect. Then, based on the N107Y_N124Y double mutation, further modifications including triple mutations N107Y_N124Y_H22W and N107Y_N124Y_M111F were performed. Among them, the N107Y_N124Y_H22W mutation is slightly less stable than the double mutation N107Y_N124Y, while the triple mutation N107Y_N124Y_M111F has enhanced thermal stability compared to the doubl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com