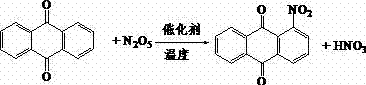Method for synthesizing 1-nitroanthraquinone by nitration of nitrogen pentoxide
A technology of dinitrogen pentoxide and nitroanthraquinone is applied in the field of dye intermediates, which can solve problems such as environmental pollution, and achieve the effects of cost reduction, less three wastes and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
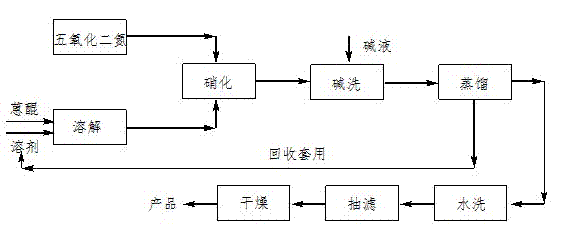
[0016] The preparation process of 1-nitroanthraquinone of the present invention is as figure 1 As shown, the equation is as follows:
[0017]
Embodiment 1
[0019] Step 1. Nitrification process:
[0020] Weigh 4.16 g (0.02 mol) of anthraquinone into a four-necked flask, add 0.48 g (0.005 mol) of methanesulfonic acid, measure 50 mL of dichloromethane and pour it into the four-necked flask, and stir Add 3.24 g (0.03 mol) of dinitrogen pentoxide, and stir the reaction at 0 °C for 1.0 h. After the reaction, neutralize with saturated aqueous sodium bicarbonate solution to pH 6~8, add a small amount of water to wash, distill off the organic solvent, filter with suction, and dry the filter cake to obtain the nitrated compound of anthraquinone, which is determined by liquid phase. The content of base anthraquinone is 86%.
[0021] Step 2. The refining of 1-nitroanthraquinone:
[0022] Weigh 2.5 g of the solid obtained in step 1, place it in a 50 mL four-neck flask, add 5 mL of N,N-dimethylformamide and 0.03 g of urea (the amount is 1.2% of the mass of nitroanthraquinone), Stir, heat to 90 °C, react for 4.0 h, cool naturally, precipitat...
Embodiment 2
[0024] Step 1. Nitrification process:
[0025] Weigh 4.16 g (0.02 mol) of anthraquinone into a four-necked flask, add 0.54 g (0.004 mol) of ferric chloride, measure 50 mL of dichloromethane and pour it into the four-necked flask, and stir Add 3.24 g (0.03 mol) of dinitrogen pentoxide, and stir the reaction at -10 °C for 2.0 h. After the reaction, neutralize with saturated aqueous sodium bicarbonate solution to pH 6~8, add a small amount of water to wash, distill off the organic solvent, filter with suction, and dry the filter cake to obtain the nitrated compound of anthraquinone, which is determined by liquid phase. The content of base anthraquinone is 80%.
[0026] Step 2. The refining of 1-nitroanthraquinone:
[0027] Weigh 2.5 g of the solid obtained in step 1, place it in a 50 mL four-neck flask, add 5 mL of N,N-dimethylformamide and 0.03 g of urea (the amount is 1.2% of the mass of nitroanthraquinone), Stir, heat to 90 °C, react for 4.0 h, cool naturally, precipitate a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com