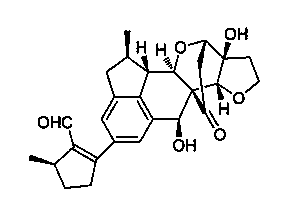
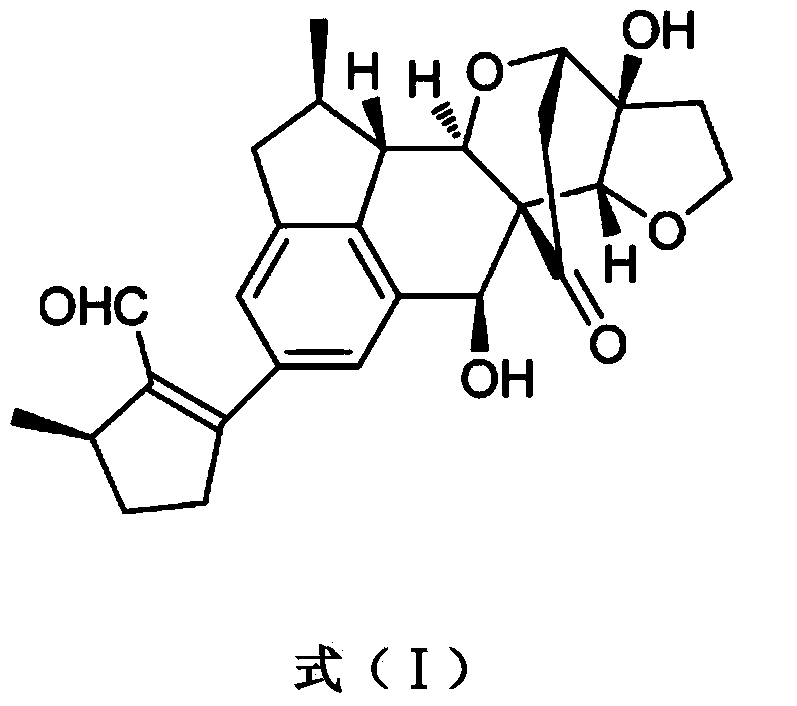
Application of Incarviatone A in anti-human fungus drug
A technology against human fungi and drugs, applied in the direction of antifungal agents, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of digestive tract irritation, strong accumulation of toxicity, etc. Active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: the preparation of compound Incarviatone A tablet involved in the present invention:
[0013] Take 20 grams of compound Incarviatone A, add 180 grams of conventional excipients for tablet preparation, mix well, and make 1000 tablets with a conventional tabletting machine.
Embodiment 2
[0014] Embodiment 2: the preparation of compound Incarviatone A capsules involved in the present invention:
[0015] Get 20 grams of compound Incarviatone A, add conventional excipients for preparing capsules such as 180 grams of starch, mix well, and pack into capsules to make 1000 tablets.
[0016] The following pharmacodynamic experiments will further illustrate its drug activity.
experiment example
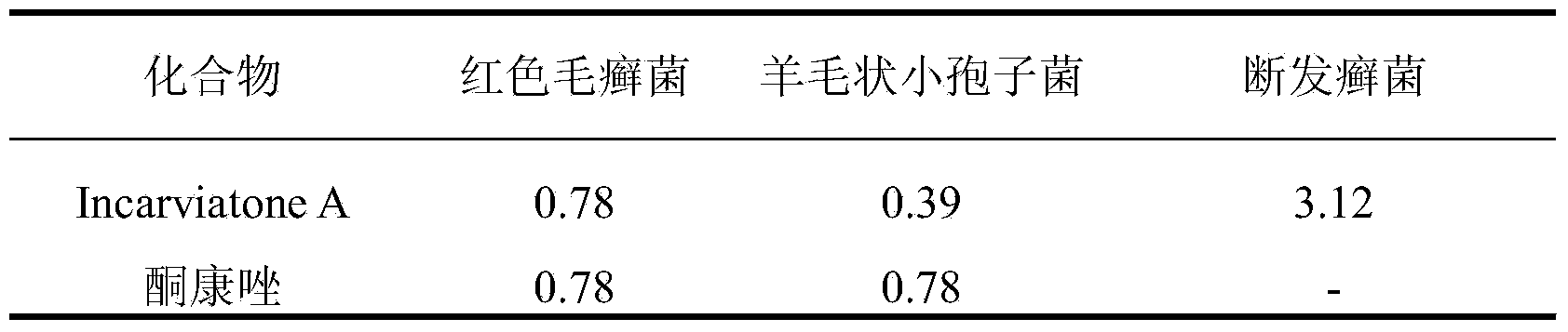
[0017] Experimental example: Incarviatone A anti-fungal activity in humans
[0018] Anti-human fungus activity experiment is the method of concentration dilution, each determination is repeated three times, the test pathogens include Trichophyton rubrum, Microsporum lanoides and Trichophyton cleavus, and the concentration of bacteria solution is 105 / mL. The initial concentration of Incarviatone A is 50.0 μg / mL (5% dimethyl sulfoxide DMSO), serially diluted to 0.098 μg / mL, and the equal volume of bacterial liquid and test samples are mixed and cultured in a 96-well plate, and the human fungal culture temperature 28°C respectively, observe after 24 hours of incubation time, if no colonies are found, it is the lowest anti-human fungal concentration of the sample, that is, the MIC value. The positive control of this experiment is ketoconazole, and the results of Incarviatone A against human fungi are shown in Table 1.
[0019] Table 1 Incarviatone A anti-human fungal MIC value (μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com