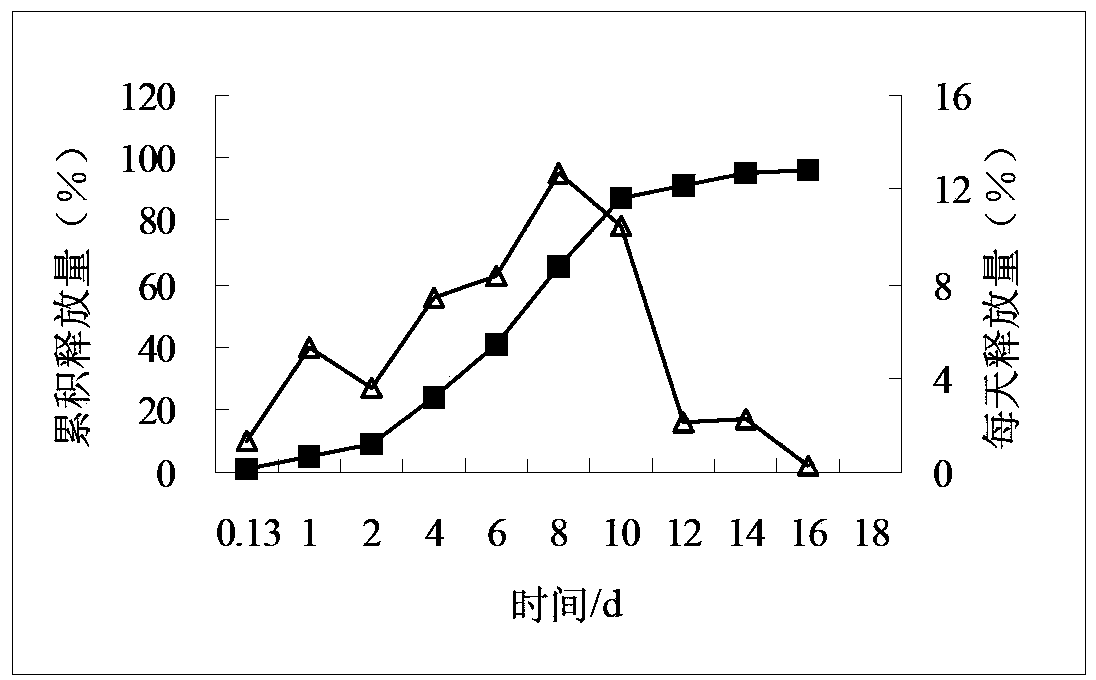
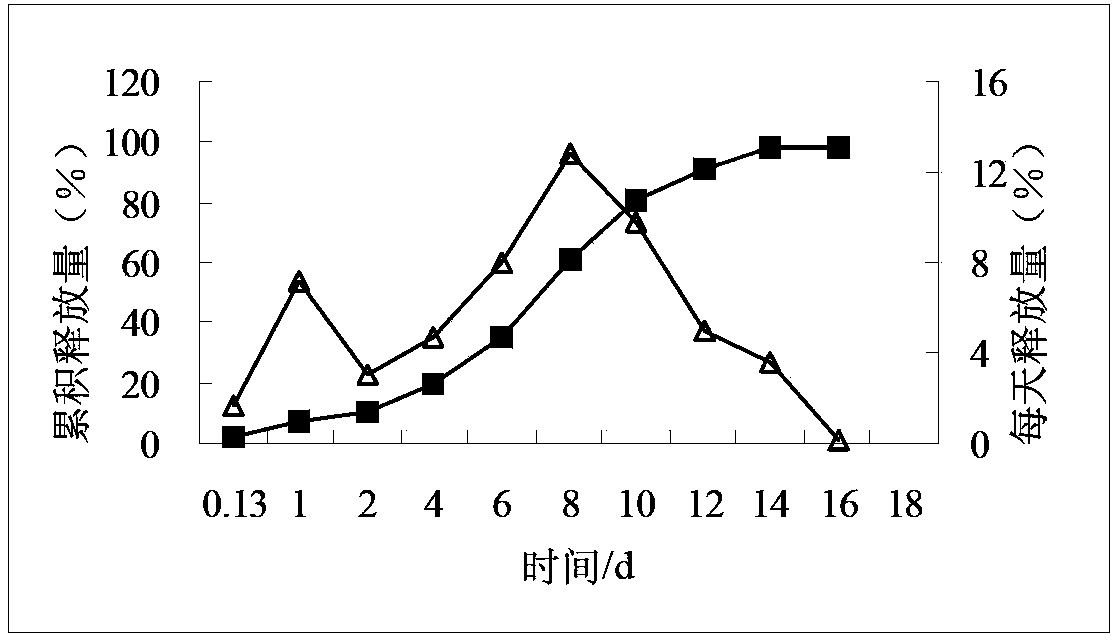
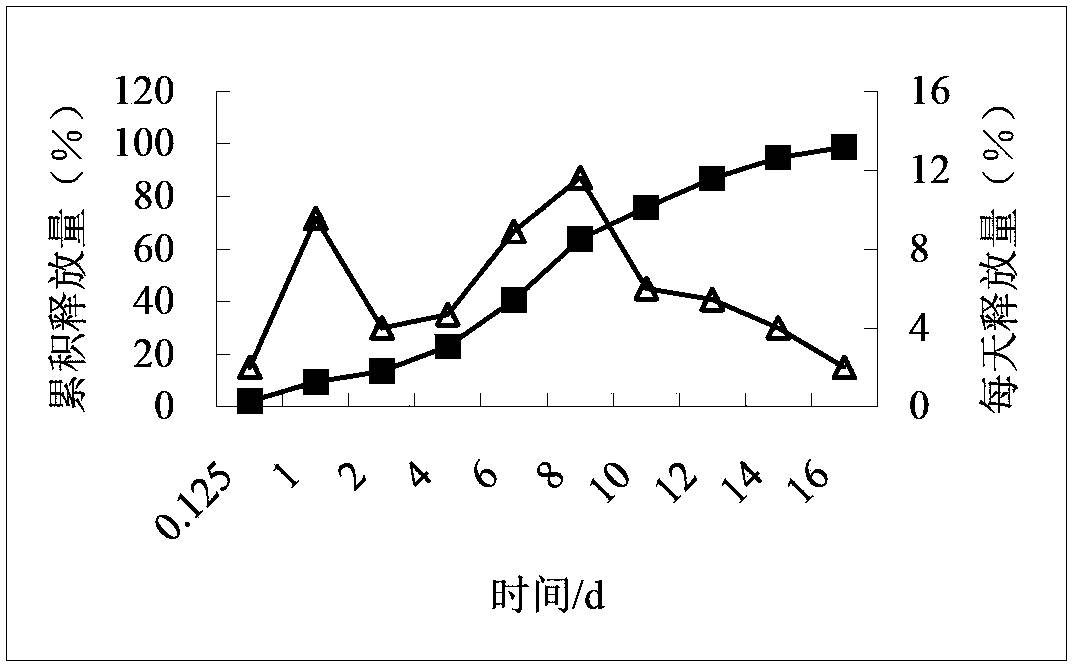
Compositions of rotigotine, derivatives thereof, or pharmaceutically acceptable salts of rotigotine or its derivative
A composition and medicinal salt technology, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-active ingredients, etc., can solve the problems of patients' pain, sudden release, drug overdose, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Example 1 Preparation of microspheres with a single PLGA (theoretical drug loading 20%)
[0138] Weigh 0.3104g of rotigotine and PLGA75254A1.2083g, add 7.5ml of dichloromethane and stir to dissolve, add to 750ml of 0.5% PVA aqueous solution with stirring (1200-2000rpm) and emulsify for 2 minutes, Reduce the stirring speed to evaporate the solvent for 5 hours, filter the obtained solution through a 1200-mesh sieve to collect the microspheres, wash the microspheres on the 1200-mesh sieve with purified water for 3 to 5 times, freeze-dry, and sieve through a 100-mesh sieve to obtain the final of microspheres.
Embodiment 2
[0139] Example 2 Preparation of microspheres with a single PLGA (theoretical drug loading 25%)
[0140] Weigh 0.3752 g of rotigotine and 1.1291 g of PLGA75254A, and prepare according to the method of Example 1 to obtain rotigotine microspheres.
Embodiment 3
[0141] Example 3 Preparation of microspheres from a single PLGA (theoretical drug loading 30%)
[0142] Weigh 0.4522 g of rotigotine and 1.0511 g of PLGA75254A, and prepare according to the method in Example 1 to obtain rotigotine microspheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com