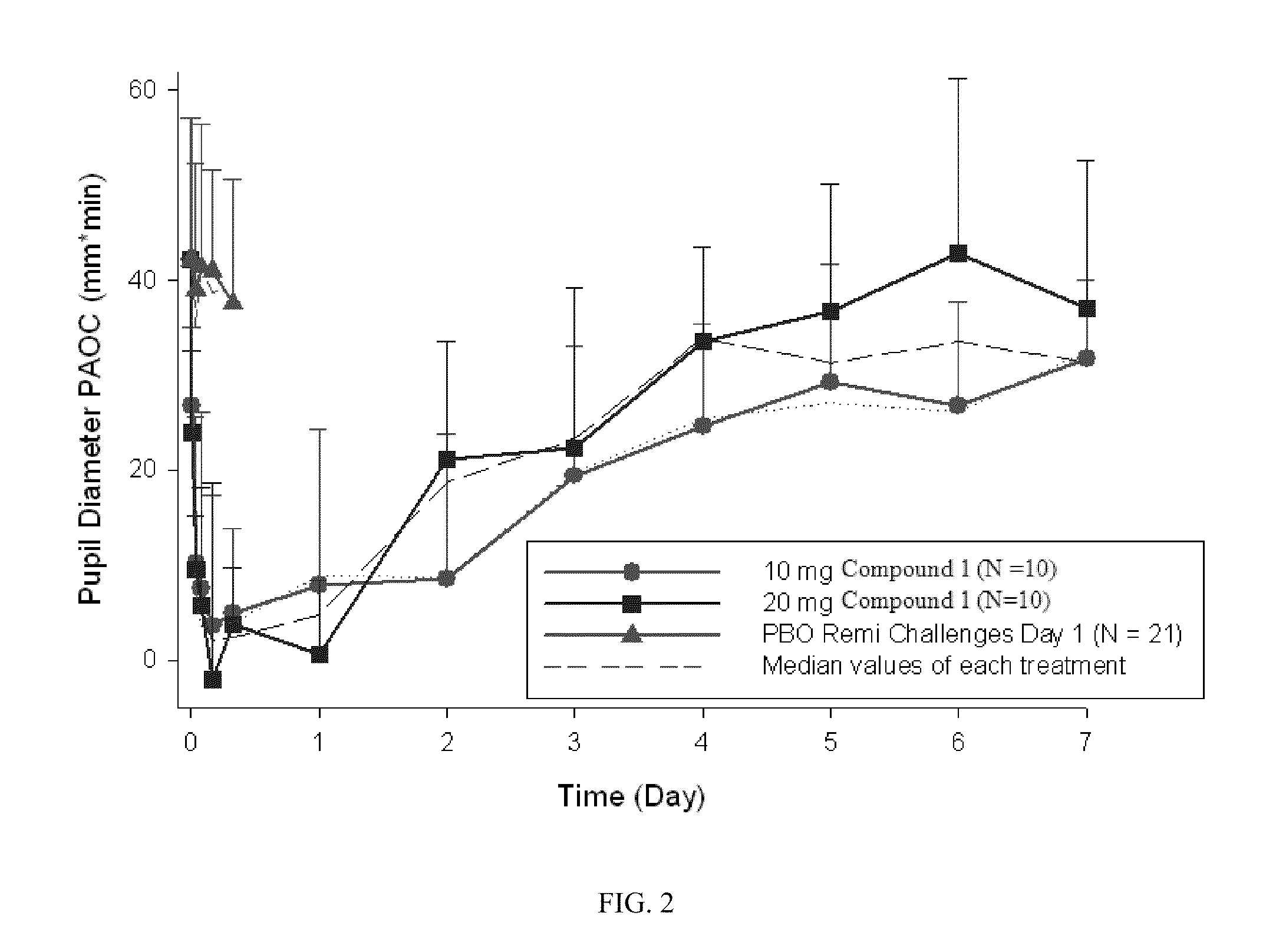
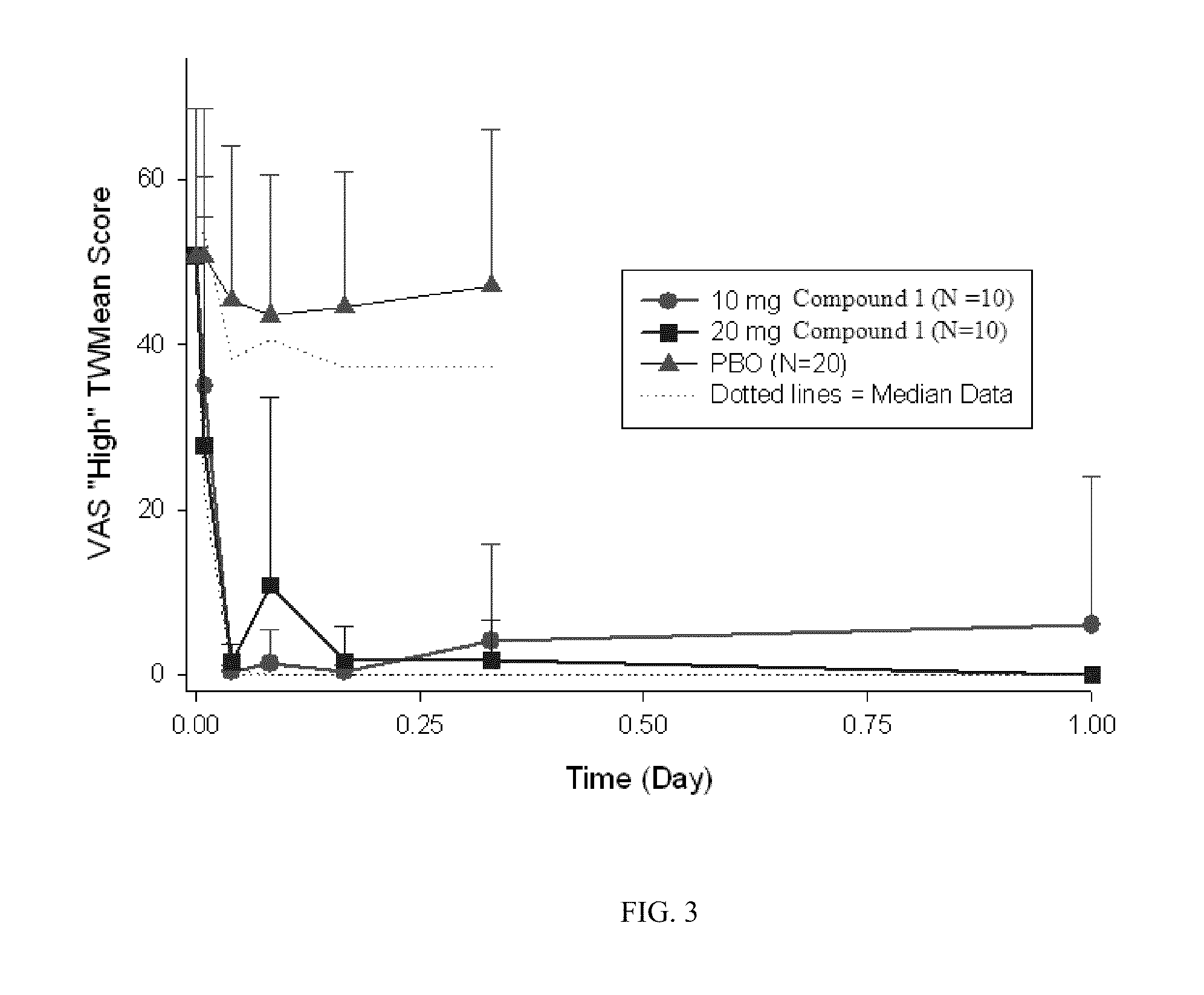
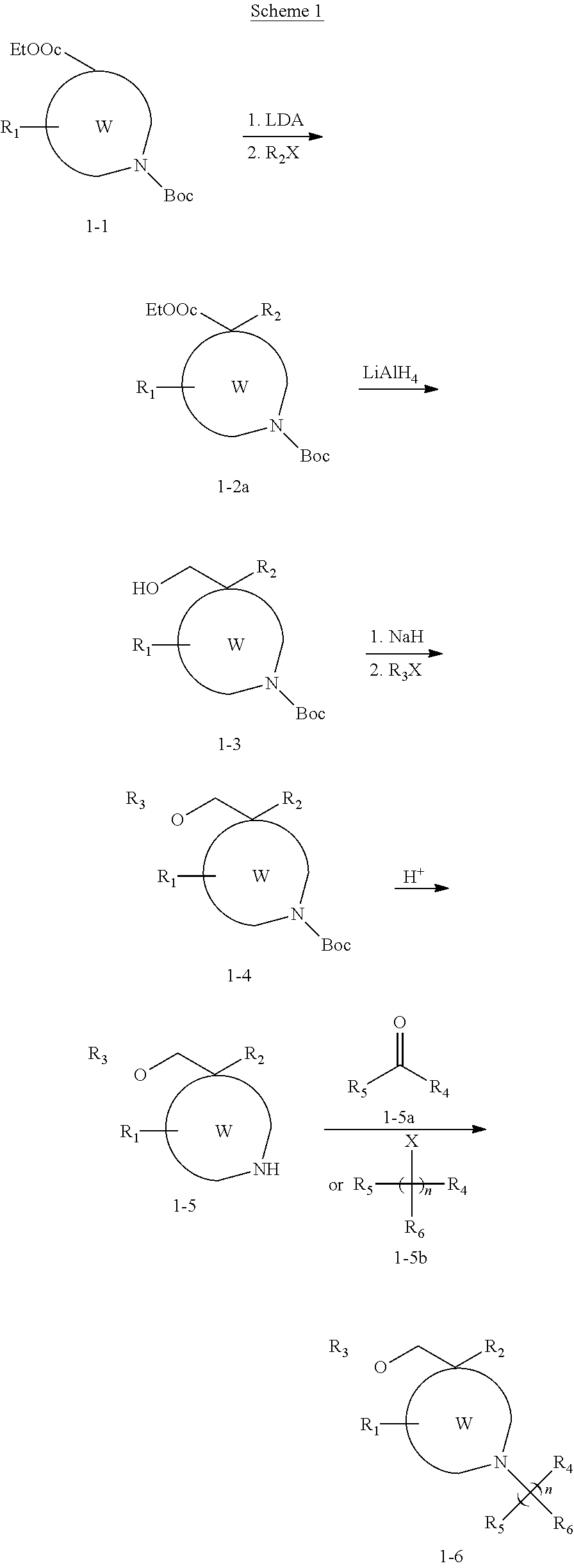
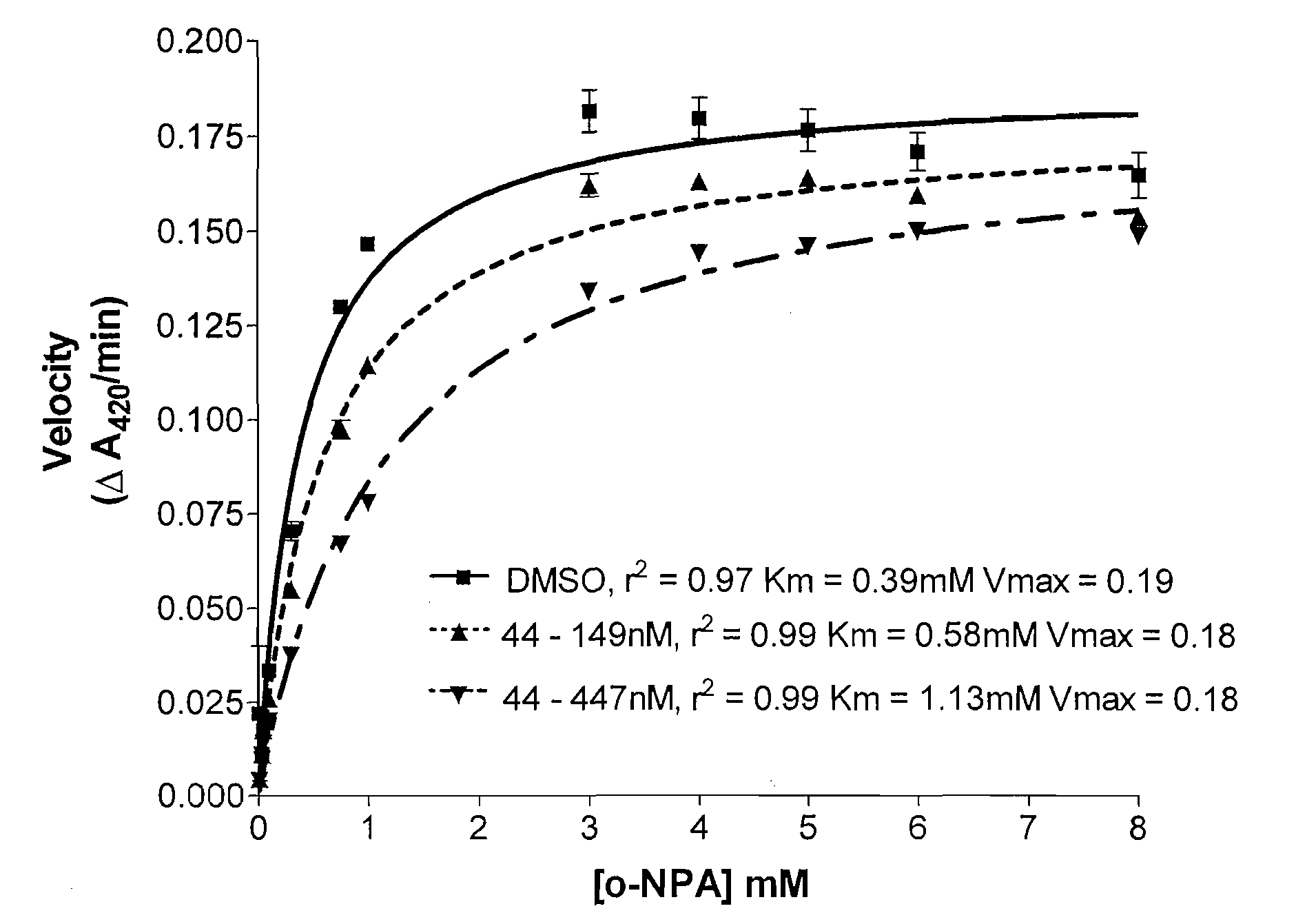
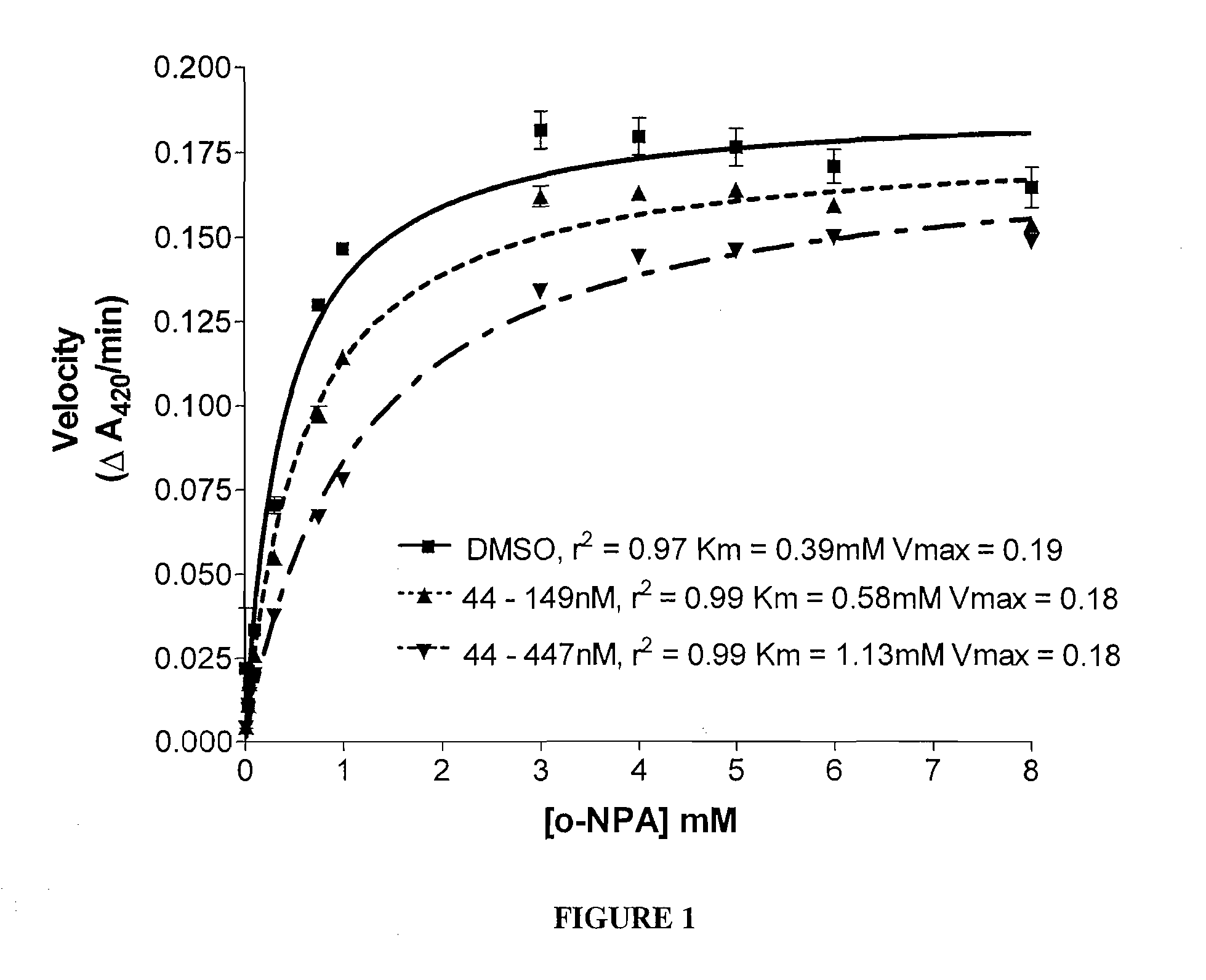
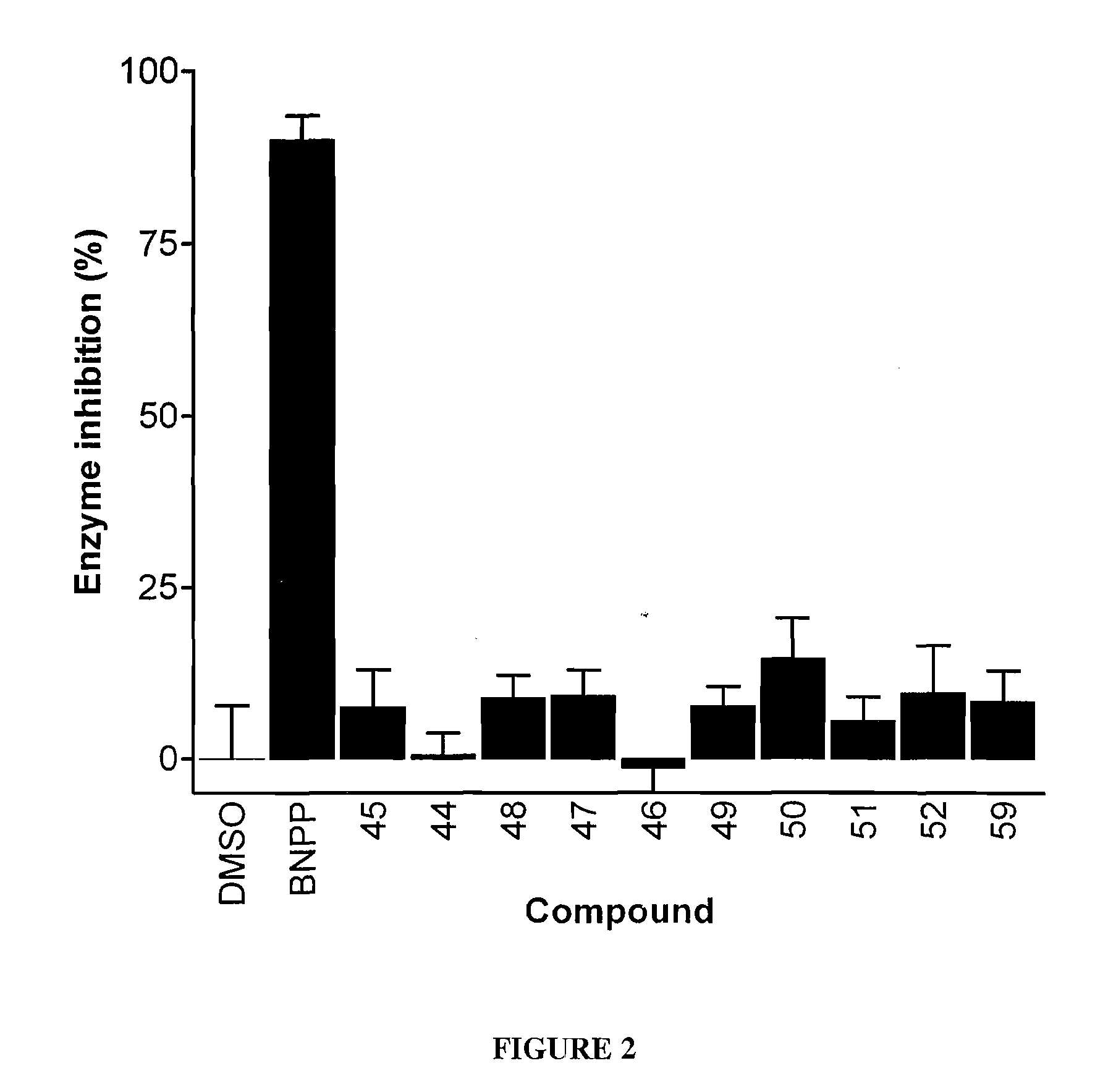
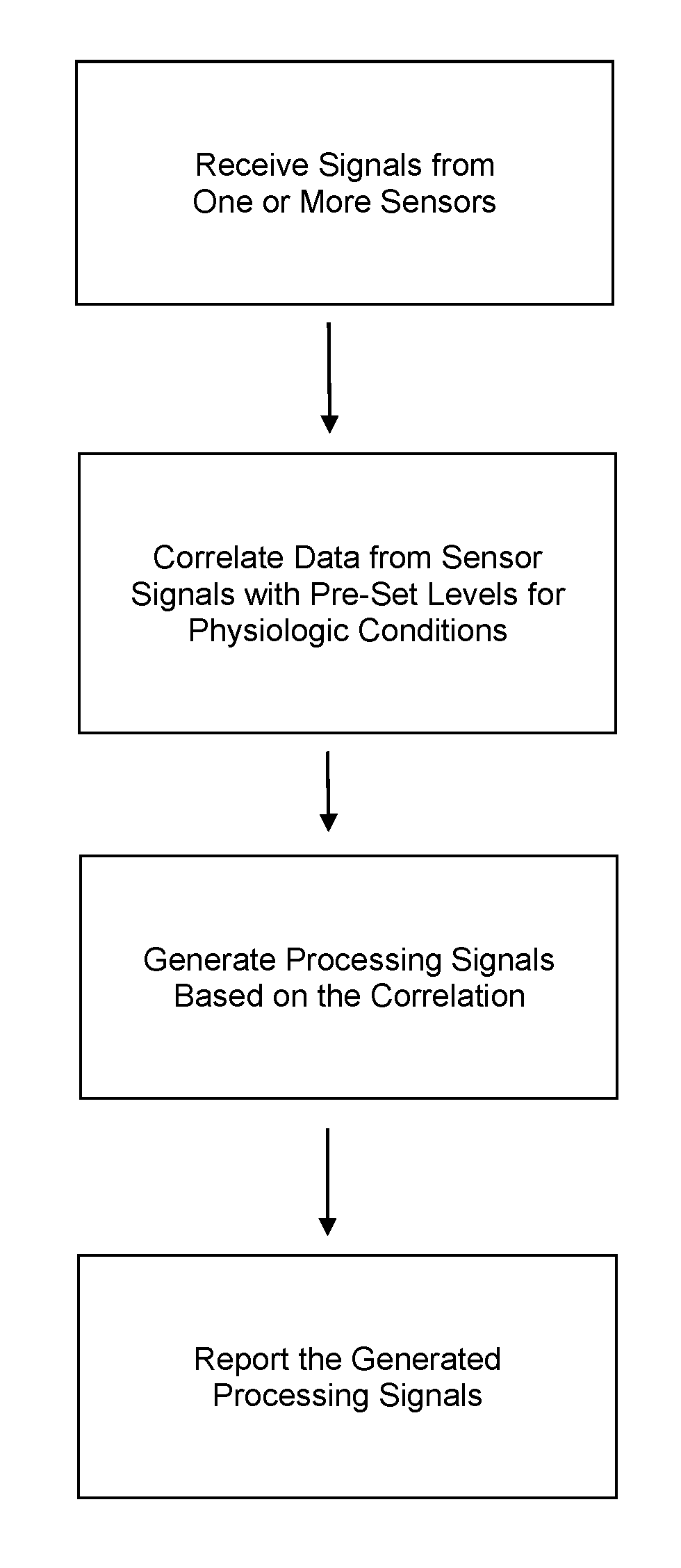
Patents
Literature
35 results about "Drug overdose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A drug overdose (or simply overdose or OD) is the ingestion or application of a drug or other substance in quantities greater than are recommended. Typically it is used for cases when a risk to health will potentially result. An overdose may result in a toxic state or death.
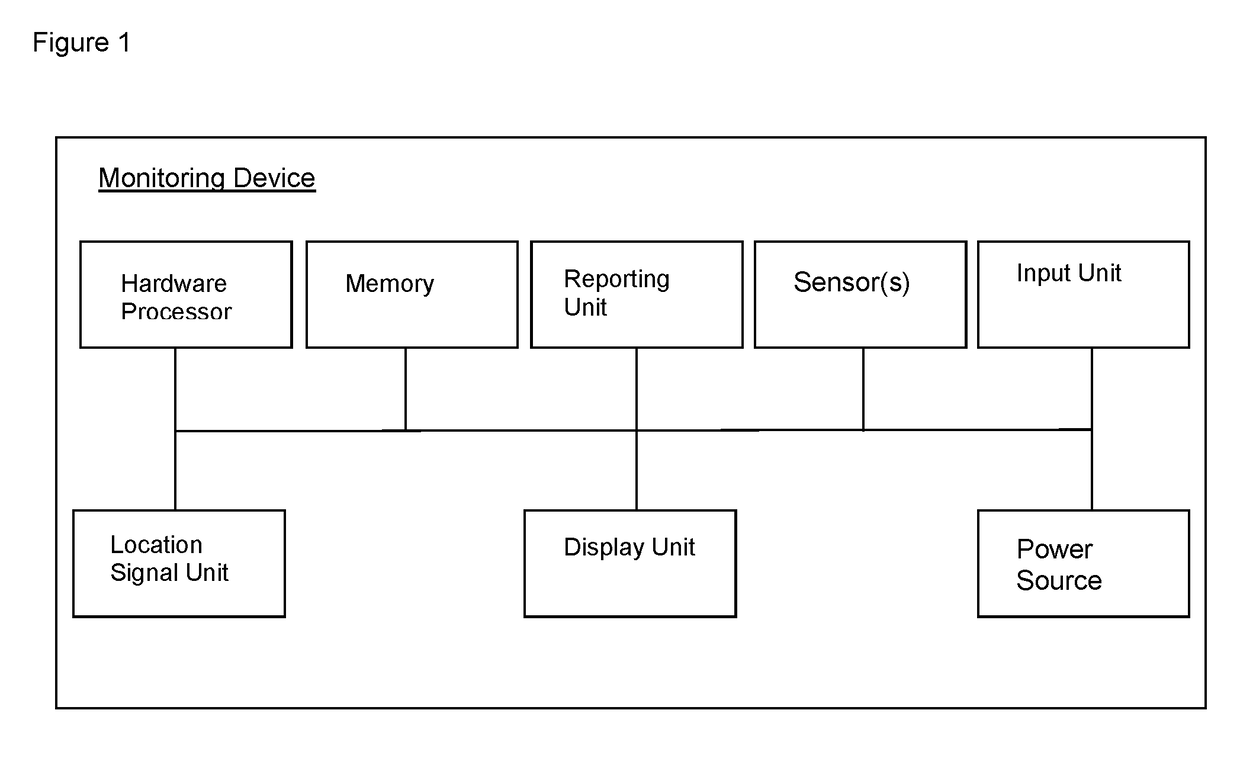
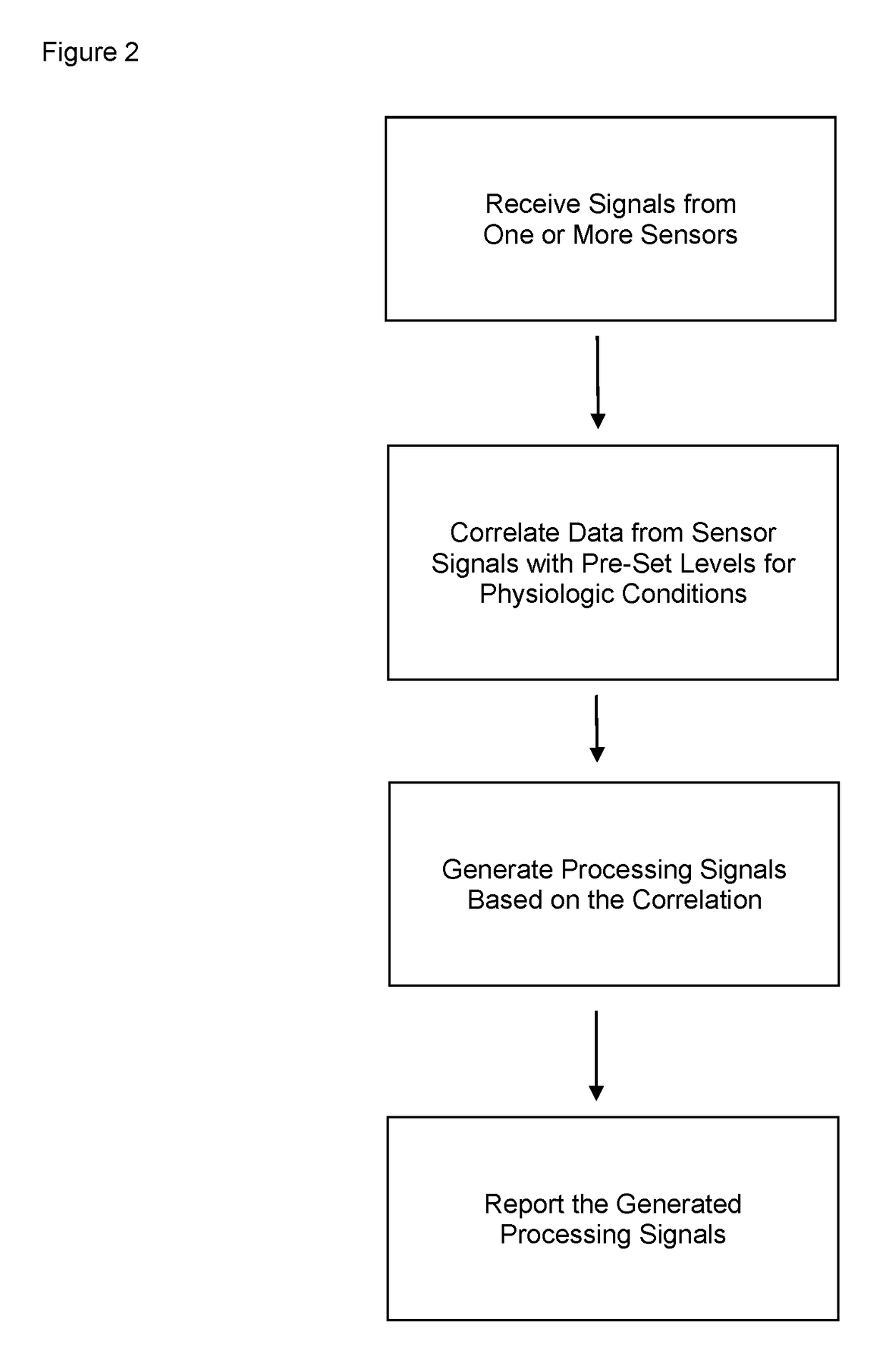
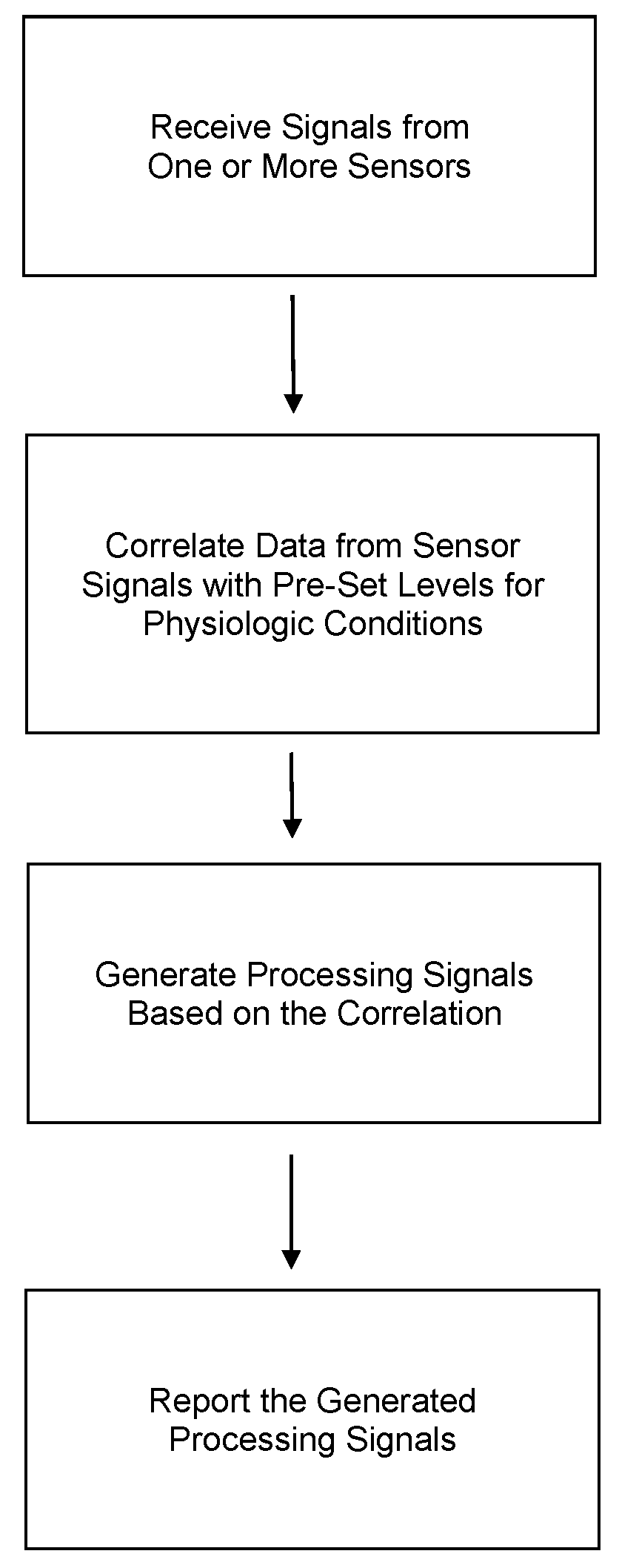
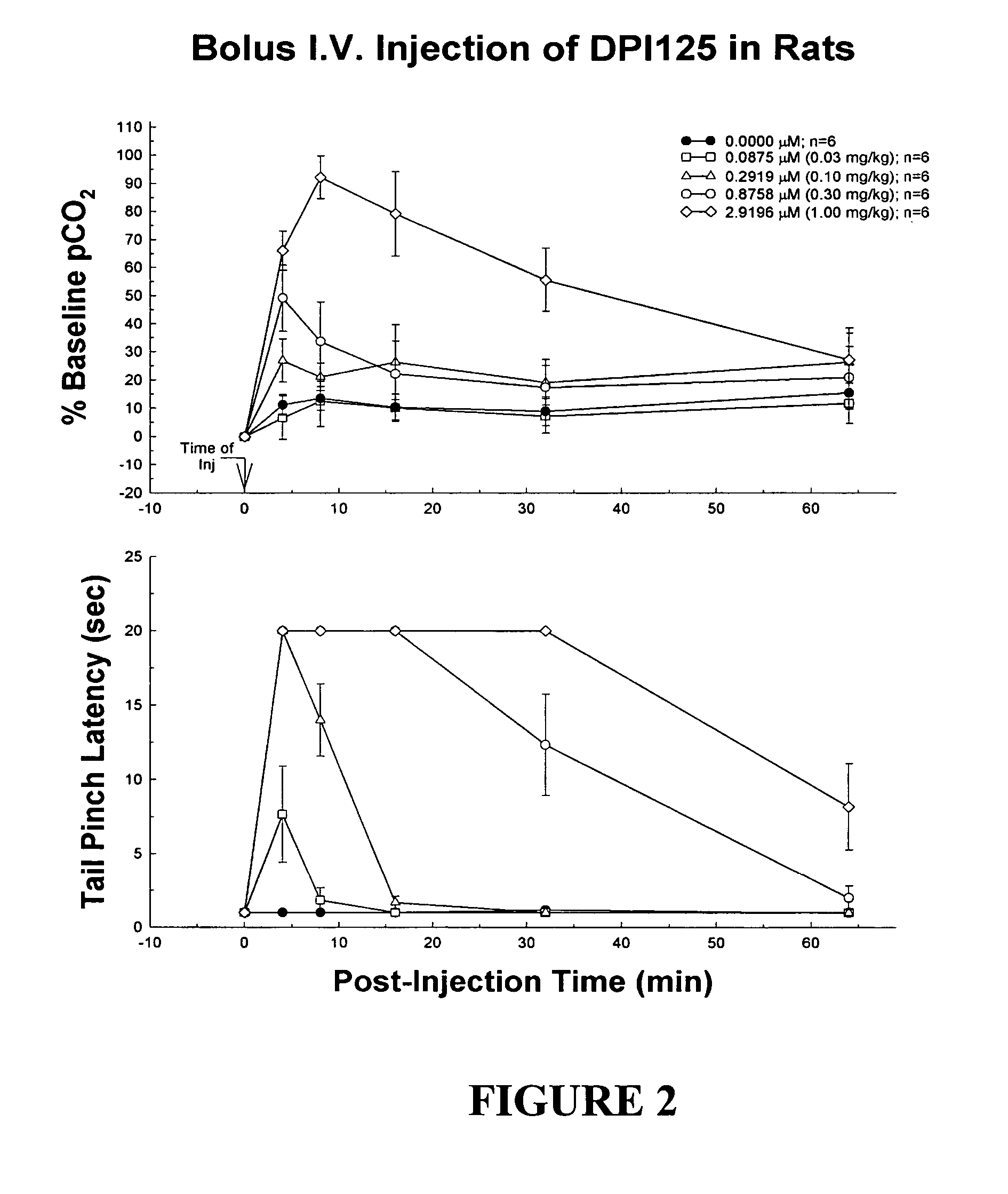
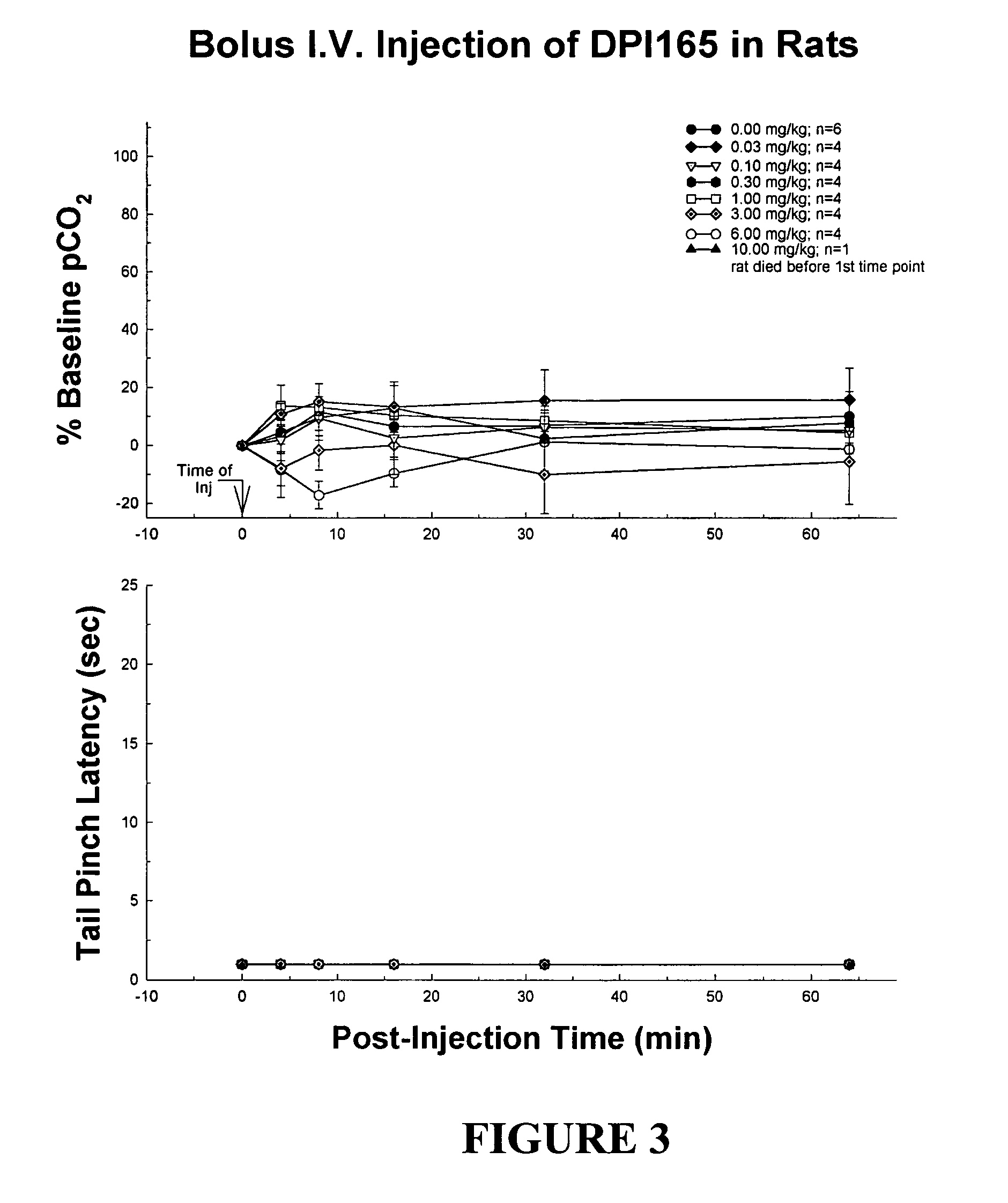
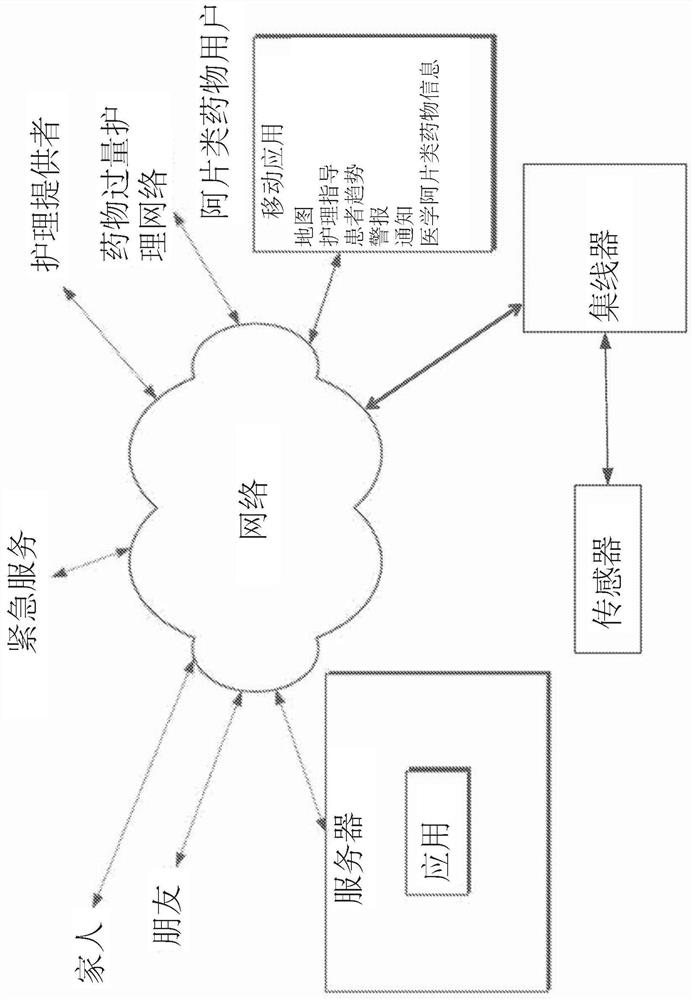
Portable patient devices, systems, and methods for providing patient aid and preventing medical errors, for monitoring patient use of ingestible medications, and for preventing distribution of counterfeit drugs
InactiveUS20080303638A1Easy maintenancePrevent kidney toxicityFinanceDrug and medicationsTransport medicineDrug dispensing
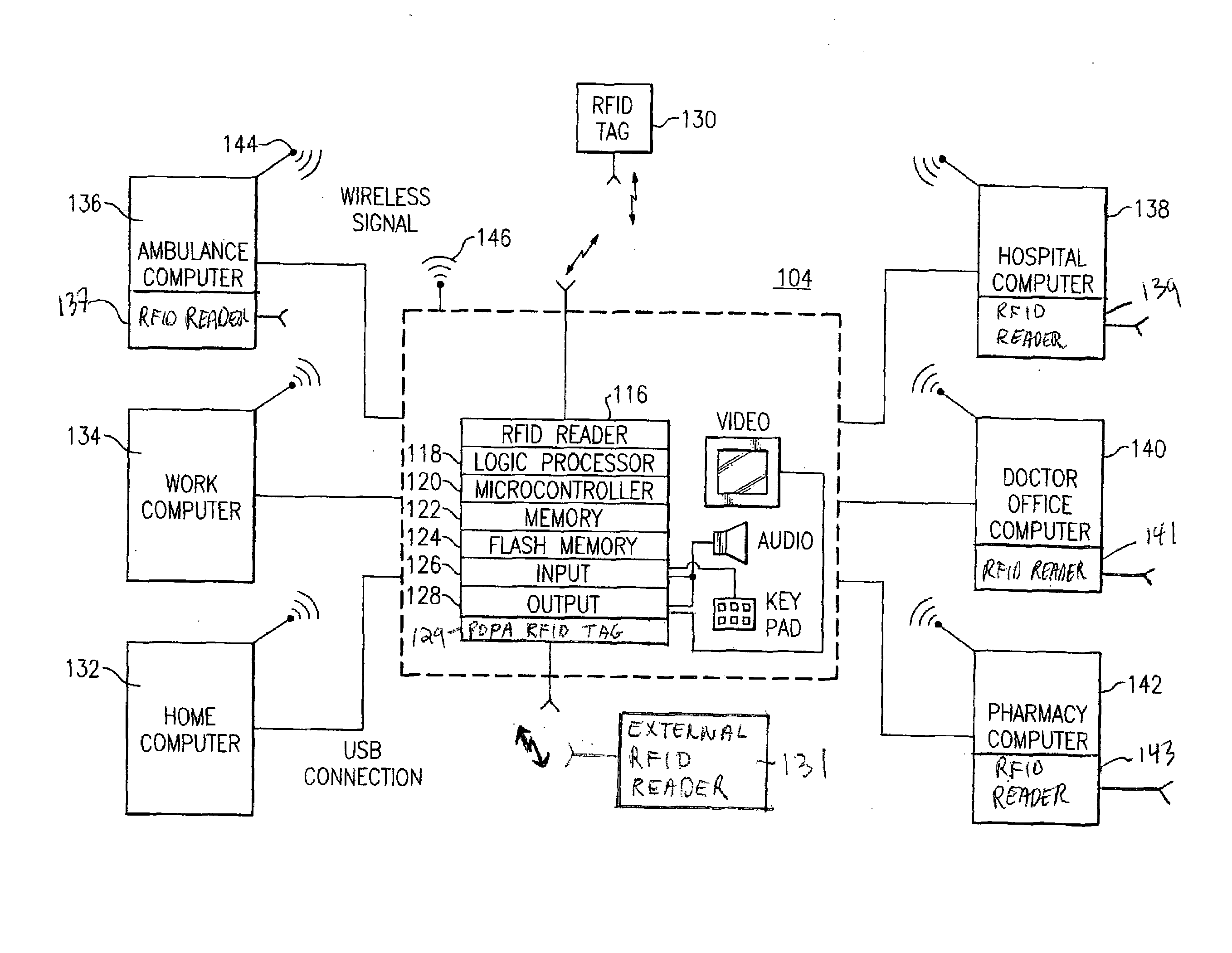
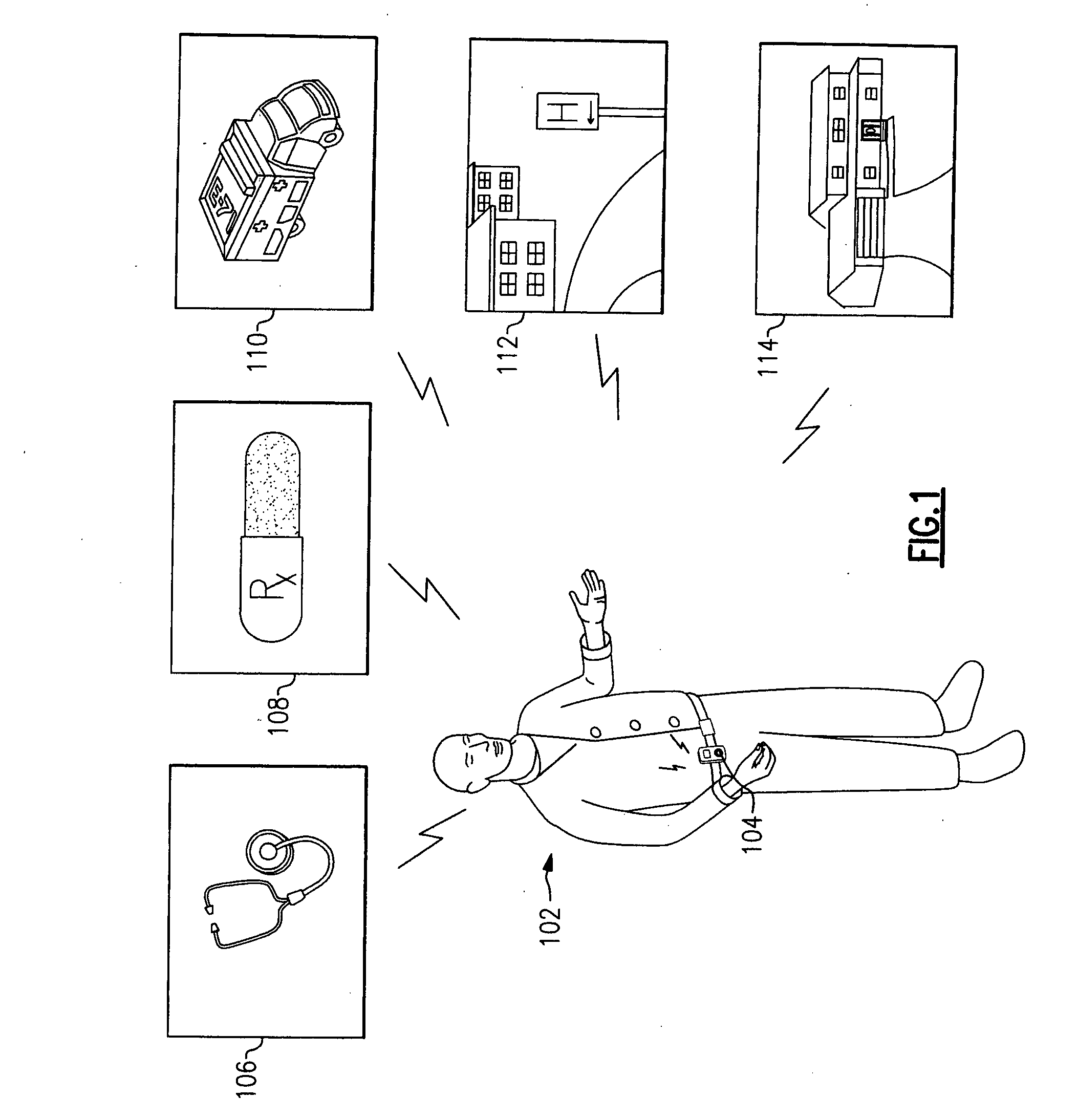
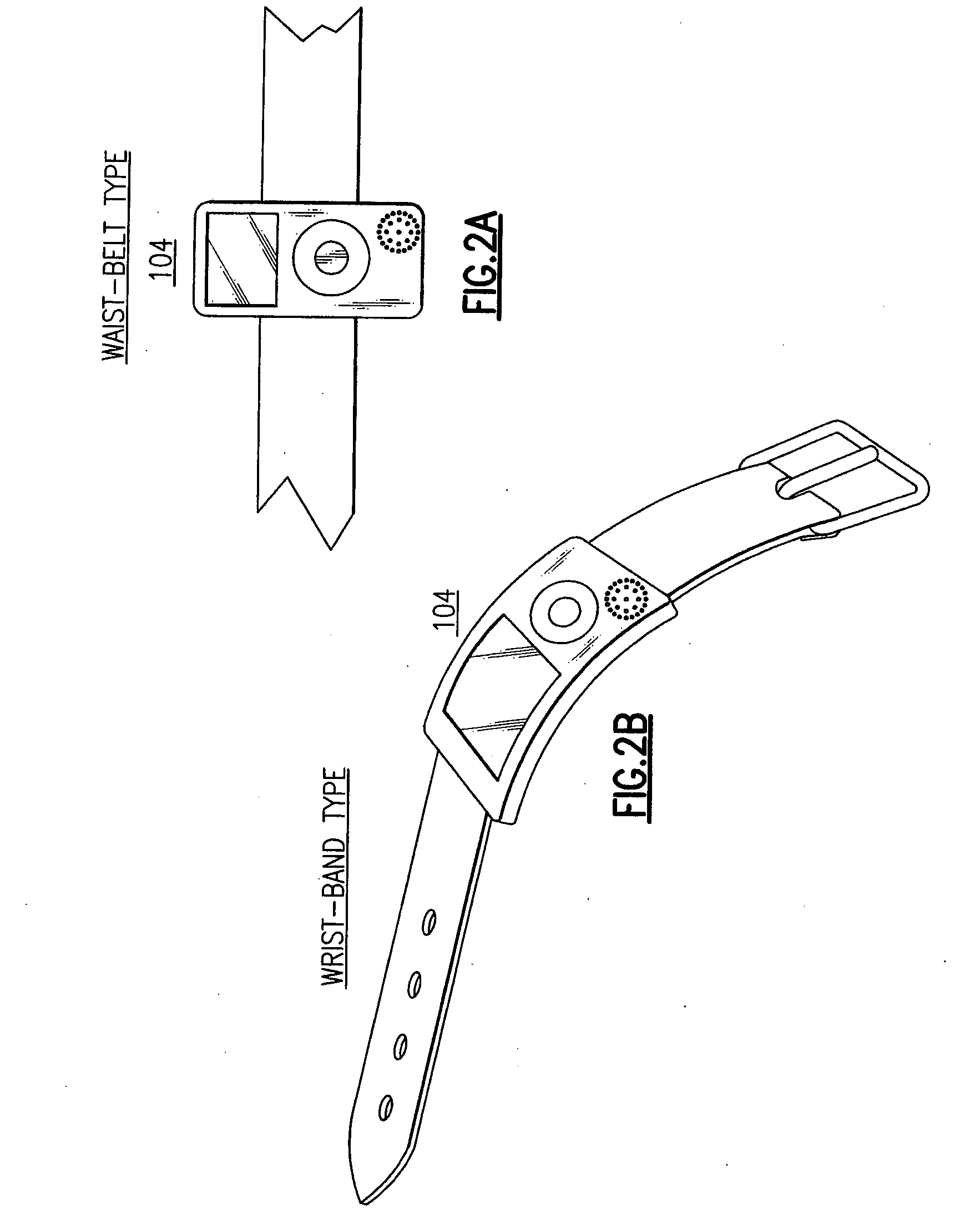
A portable digital patient assistant includes an RFID reader, a central processing unit for processing signals received from the RFID reader, a memory for storing data, and an output operatively linked to the central processing unit for providing output information regarding use of medicinal drugs. Methods for using the portable digital patient assistant include use at the doctor's office, pharmacy, emergency medical vehicle, hospital, home, and use while taking medications to the verify authenticity thereof and prevent drug overdoses. Related methods and systems for manufacturing, packaging, and shipping medicinal drugs to prevent the distribution of counterfeit drugs are also provided. One of the methods includes the steps of preparing a predetermined amount of a specific type of drug for patient end-users; forming discrete individual doses of the specific type of drug; and associating a respective RFID tag with each of the discrete individual doses of the specific type of drug so that when the specific type of drug is distributed to the patient end-users, at least one RFID reader may be employed to read the RFID tags associated with each of the discrete individual doses to thereby verify the authenticity of the doses as they move through a distribution channel from a manufacture to the patient end-users.
Owner:NGUYEN HAP +1
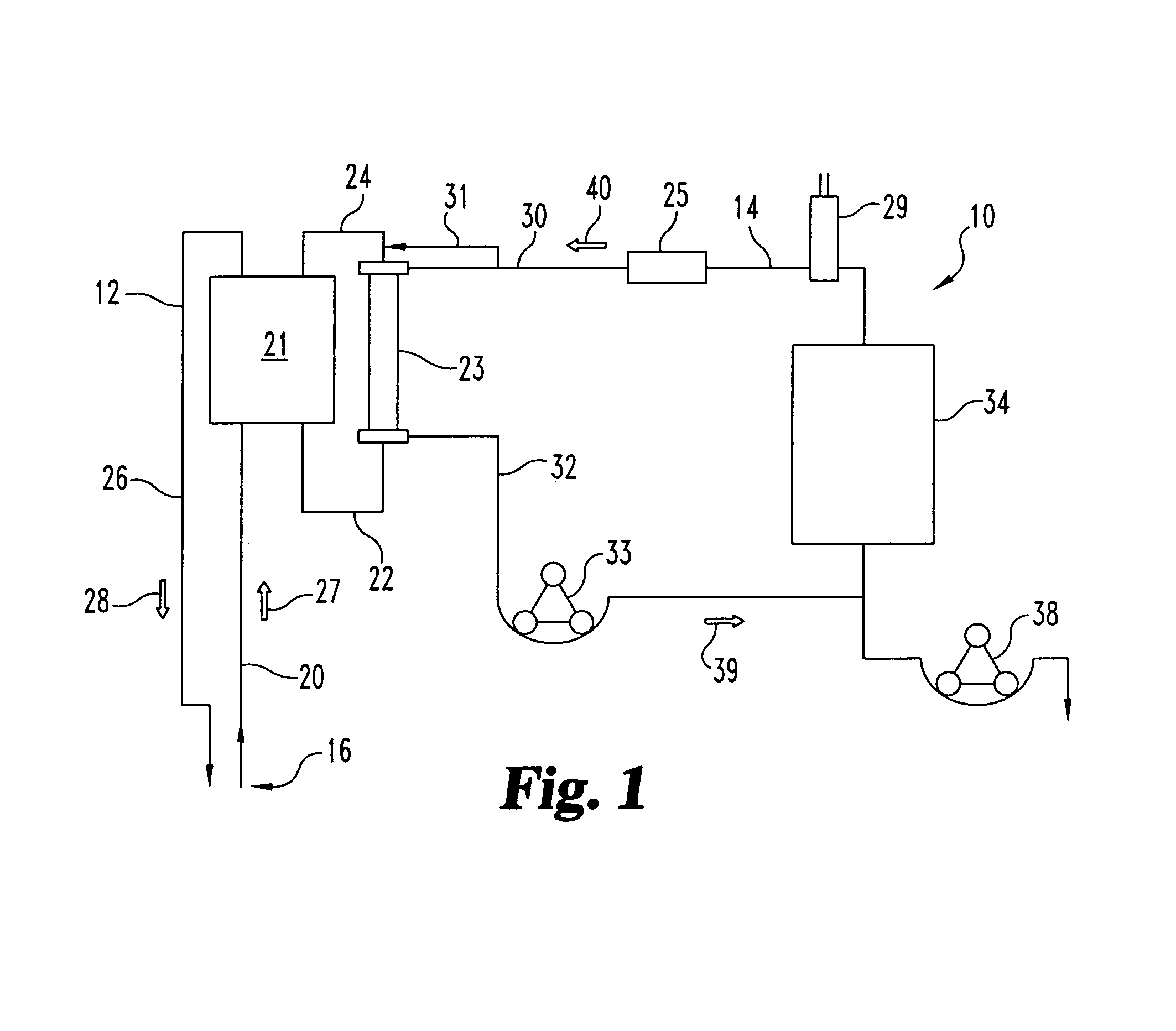
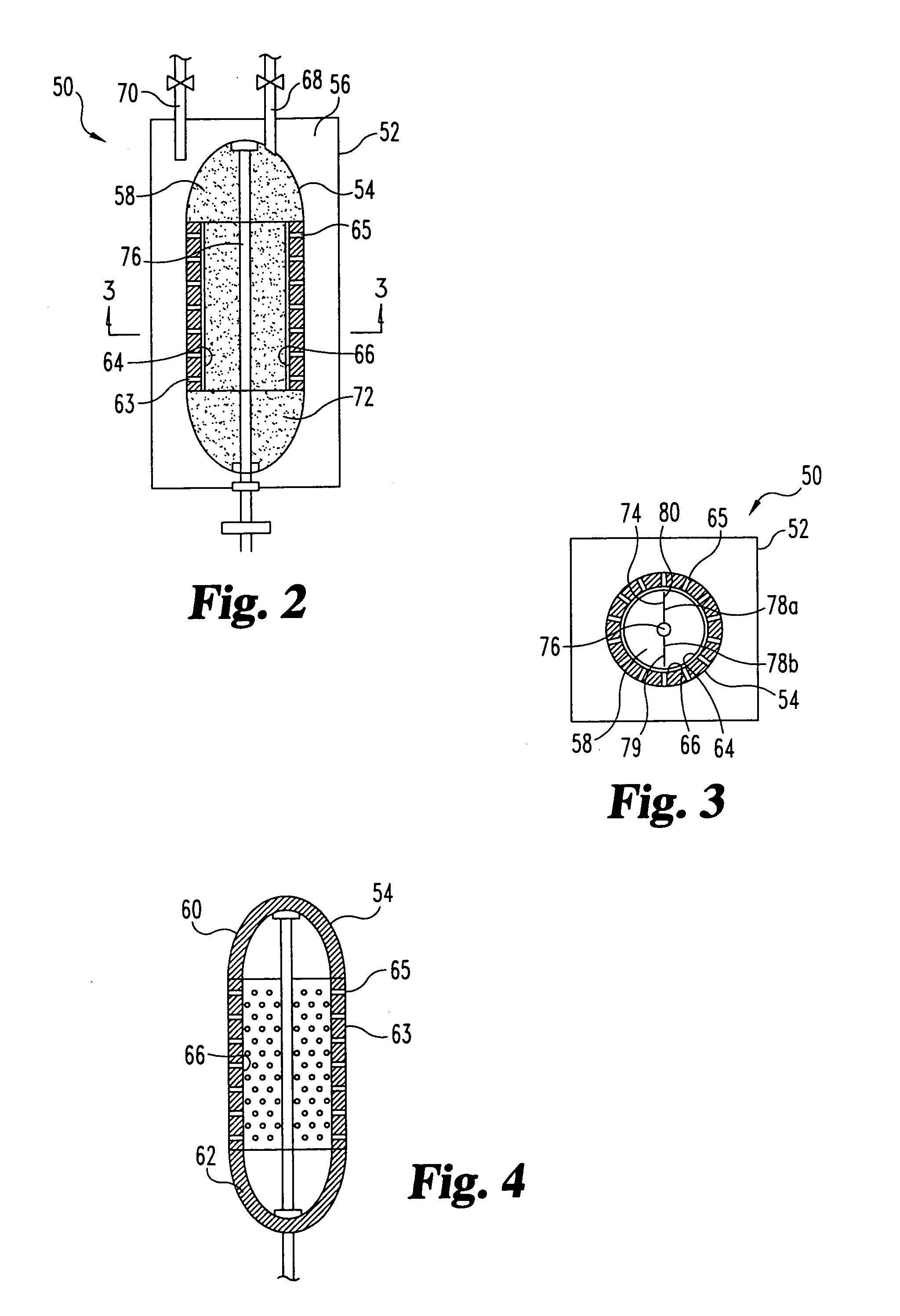
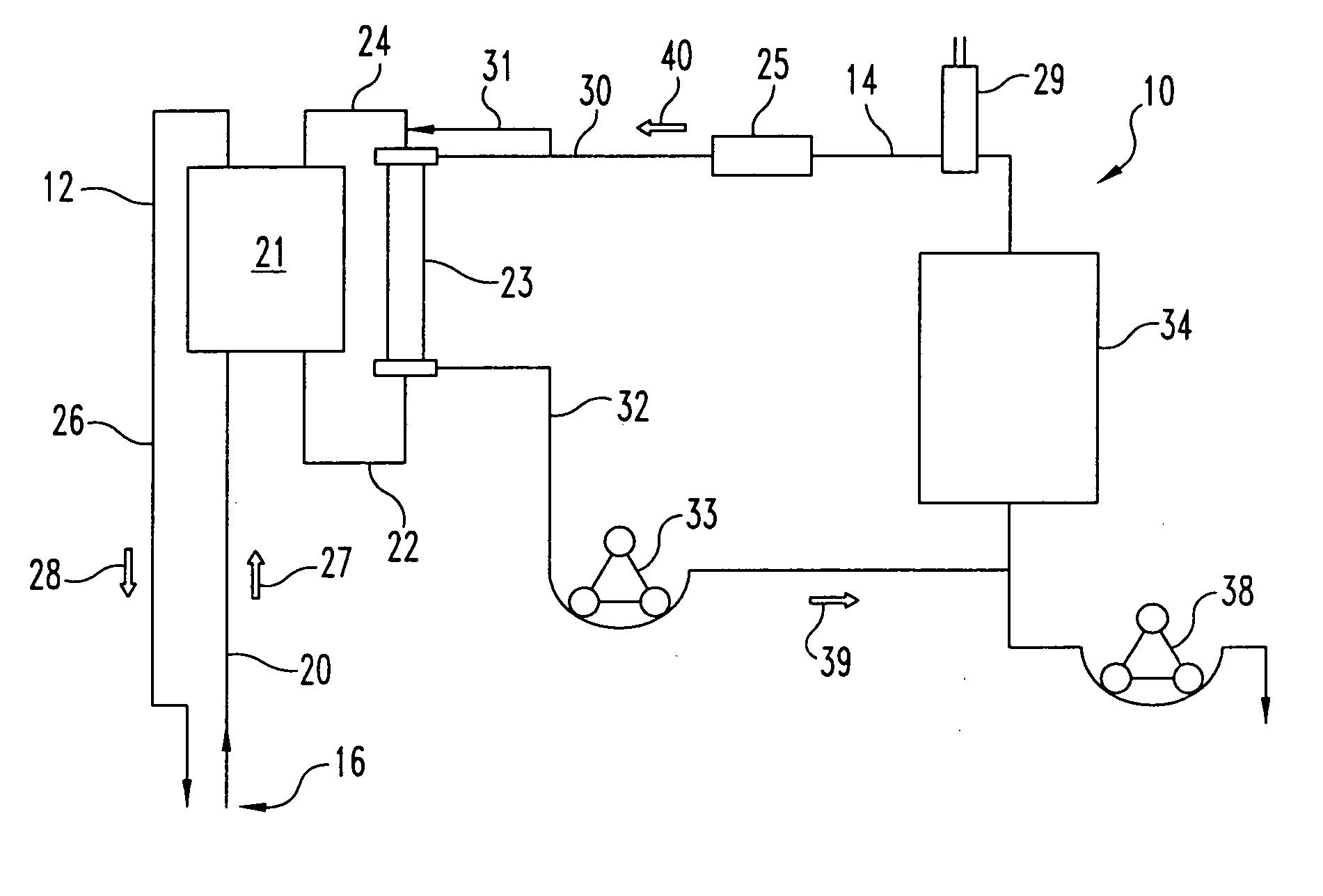
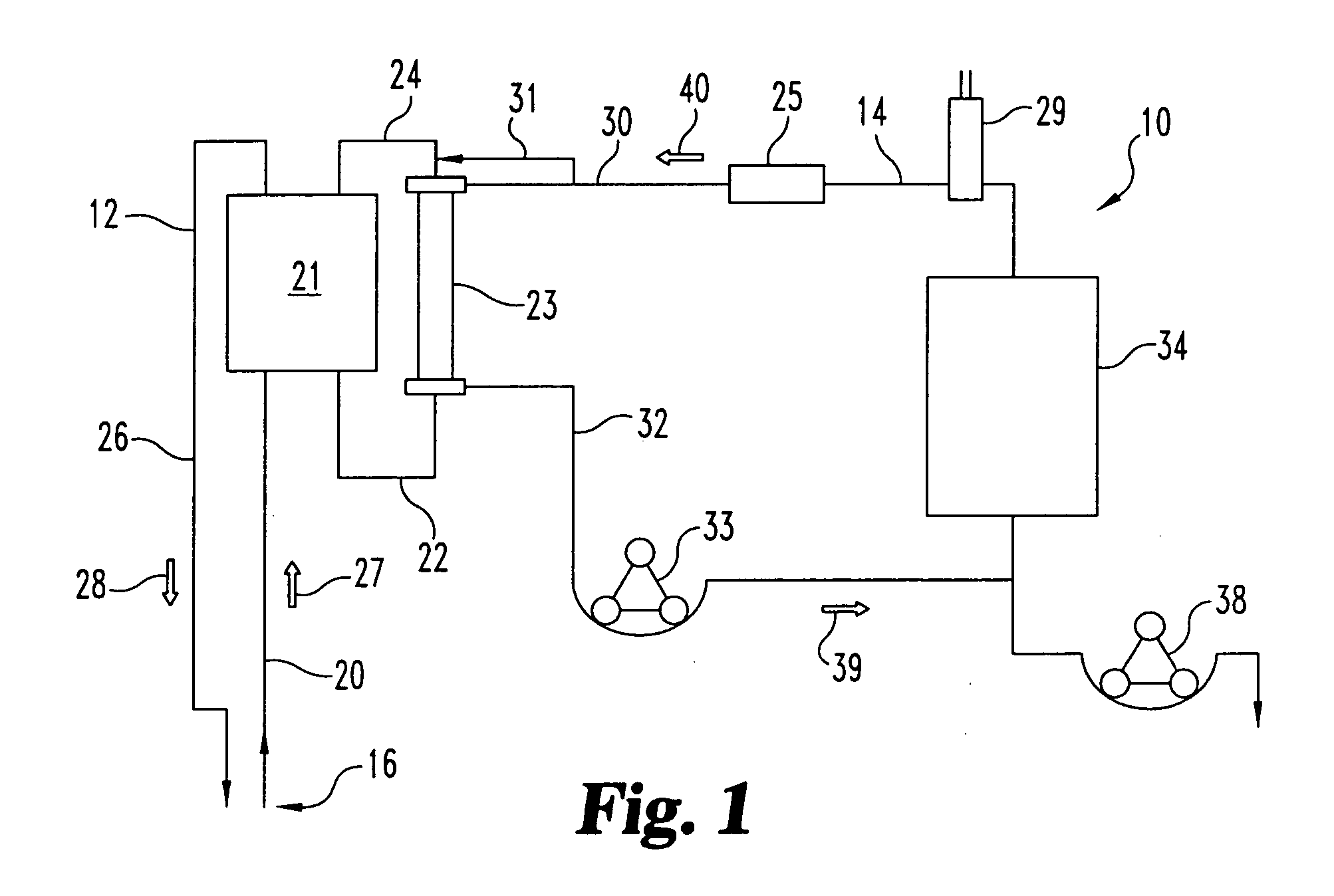
Sorbent reactor for extracorporeal blood treatment systems, peritoneal dialysis systems, and other body fluid treatment systems
InactiveUS7169303B2Facilitate homogeneous suspensionReduce probabilitySolvent extractionHaemofiltrationFluid balancePeritoneal dialysis
Systems and methods for extracorporeal processing of blood or other body fluid for the treatment of conditions, such as sepsis, autoimmune disease, or toxemia related to kidney failure, liver failure, or drug overdose are provided. In an extracorporeal treatment system, a fraction of a body fluid is passed into a treatment fluid, at least a portion of which is then passed through a sorbent suspension reactor for treatment by a sorbent suspension. The treatment fluid circuit can be maintained at a fixed volume, which enables accurate fluid balance between the patient and the extracorporeal circuit. Some or all of the treatment fluid, optionally also containing nutrients and / or therapeutic agents, is returned to the patient. In a peritoneal dialysis system, dialysate is passed into a patient's peritoneal cavity, recovered from the cavity, passed through a sorbent suspension reactor in accordance with the invention, and returned to the cavity.
Owner:HEMOCLEANSE TECH
Sorbent reactor for extracorporeal blood treatment systems, peritoneal dialysis systems, and other body fluid treatment systems
InactiveUS20050006296A1Facilitate homogeneous suspensionReduce probabilityWater treatment parameter controlSemi-permeable membranesFluid balancePeritoneal dialysis
Systems and methods for extracorporeal processing of blood or other body fluid for the treatment of conditions, such as sepsis, autoimmune disease, or toxemia related to kidney failure, liver failure, or drug overdose are provided. In an extracorporeal treatment system, a fraction of a body fluid is passed into a treatment fluid, at least a portion of which is then passed through a sorbent suspension reactor for treatment by a sorbent suspension. The treatment fluid circuit can be maintained at a fixed volume, which enables accurate fluid balance between the patient and the extracorporeal circuit. Some or all of the treatment fluid, optionally also containing nutrients and / or therapeutic agents, is returned to the patient. In a peritoneal dialysis system, dialysate is passed into a patient's peritoneal cavity, recovered from the cavity, passed through a sorbent suspension reactor in accordance with the invention, and returned to the cavity.
Owner:HEMOCLEANSE TECH
Selective opioid compounds
ActiveUS20090209569A1Reducing lipid permeability of drugReduce penetrationAntibacterial agentsBiocideDiseaseInterstitial cystitis
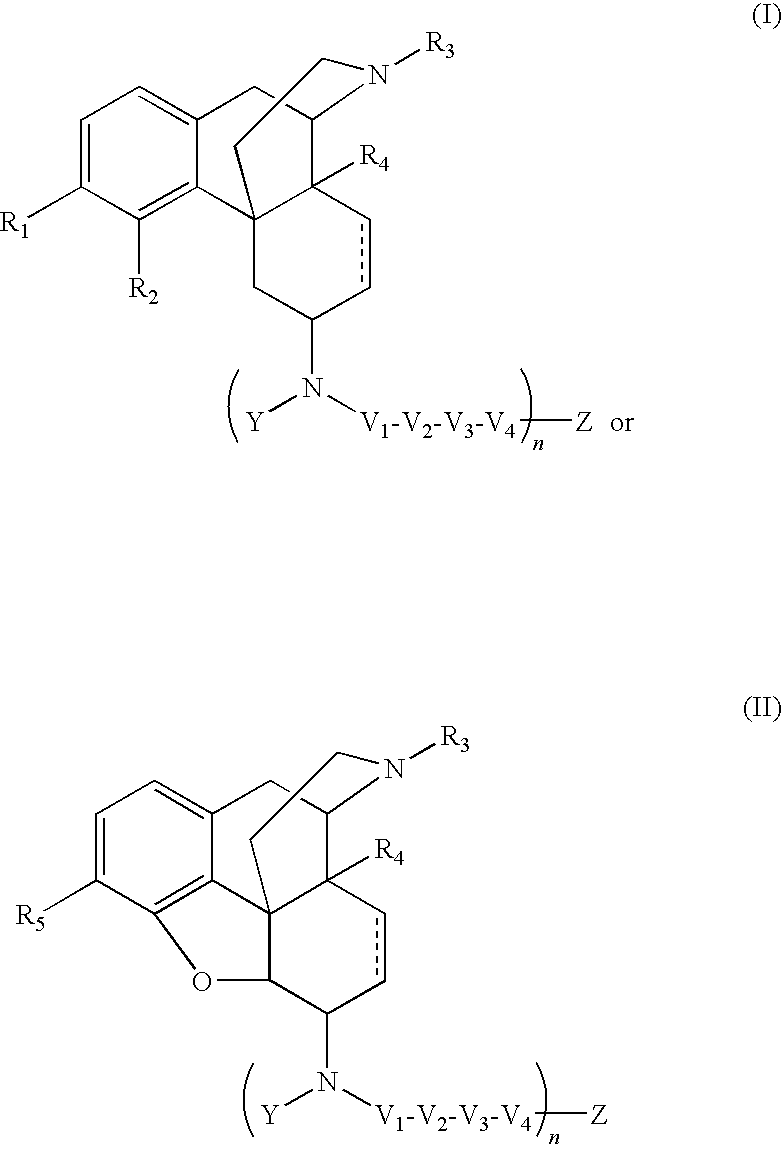
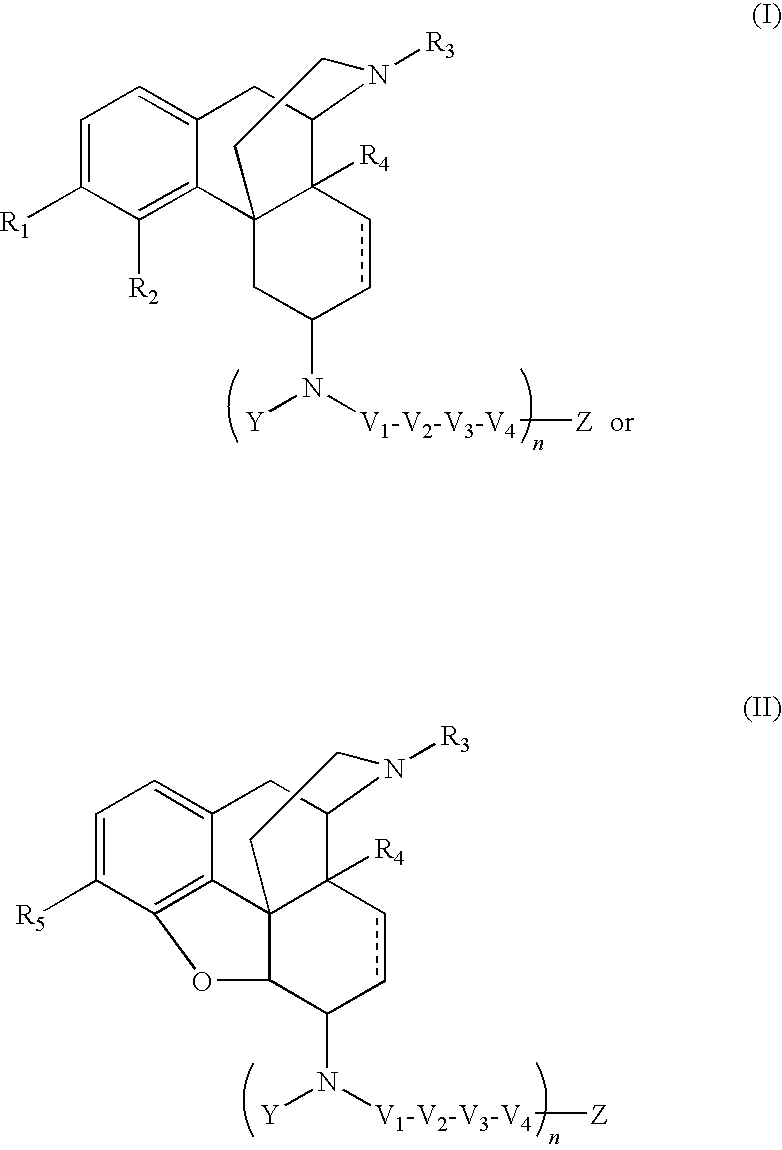
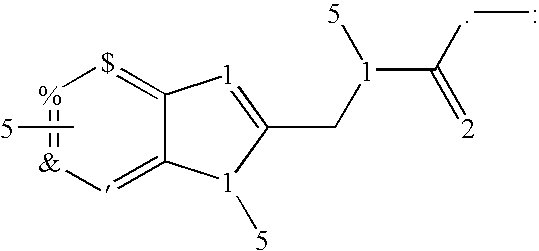
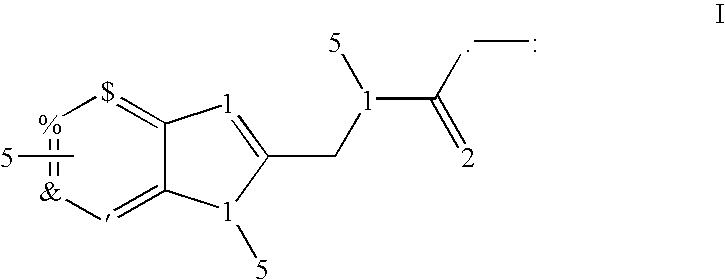
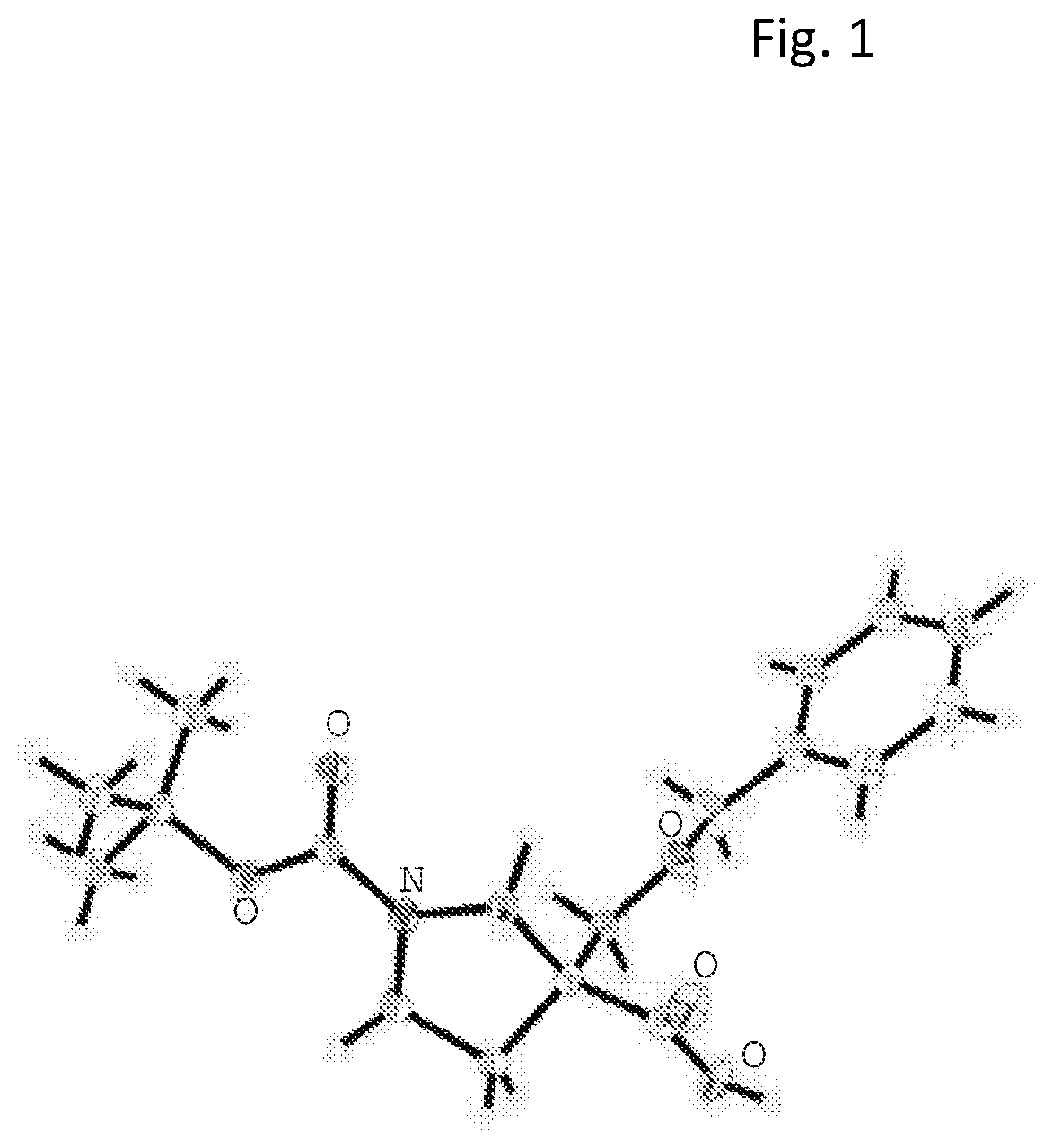
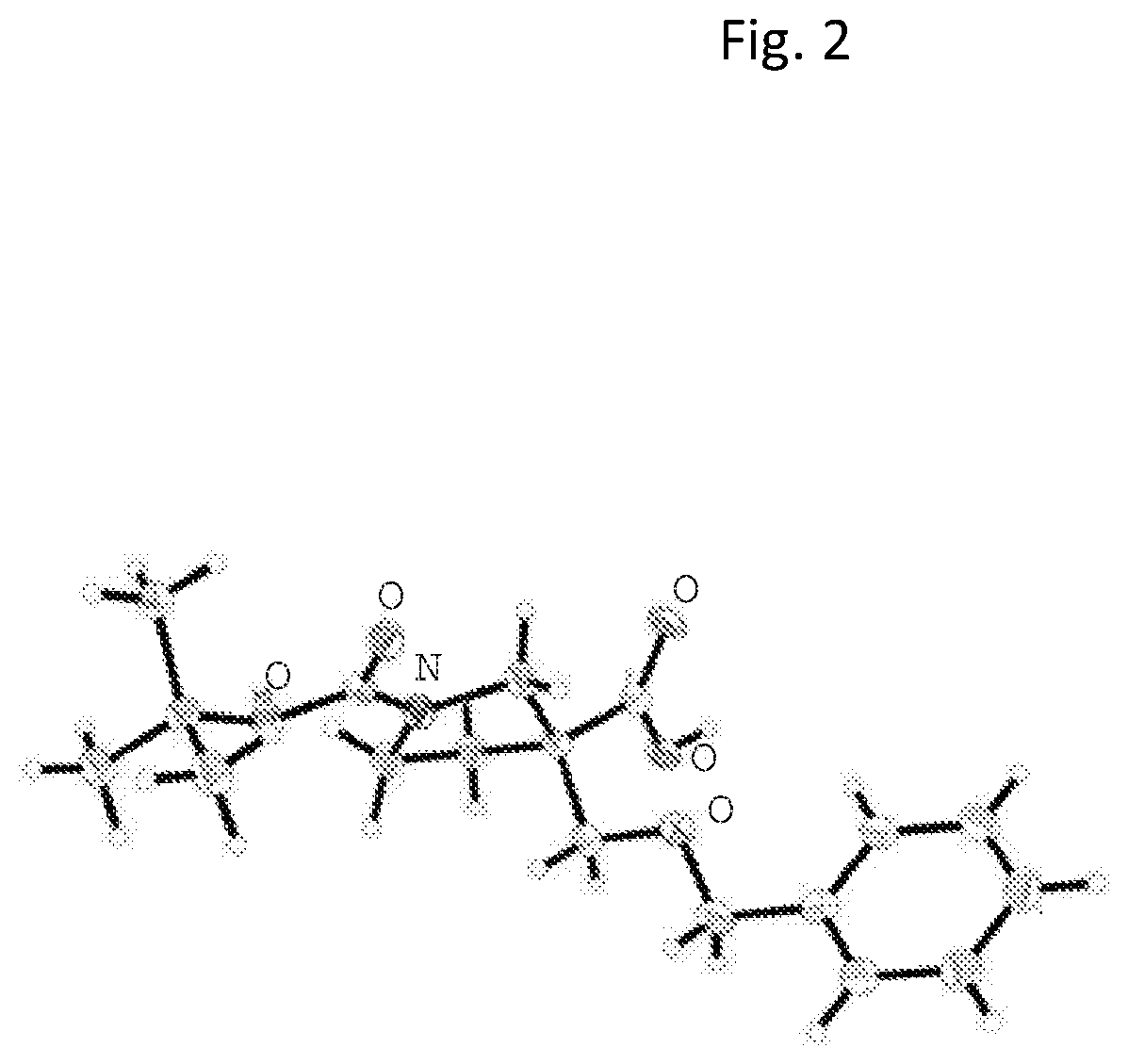
The present invention relates to compounds of Formula I or II, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which relates to morphinan compounds useful as μ, δ, and / or κ receptor opioid compounds and pharmaceuticals containing same that may be useful for mediating analgesia, combating drug addiction, alcohol addiction, drug overdose, mental illness, bladder dysfunctions, neurogenic bladder, interstitial cystitis, urinary incontinence, premature ejaculation, inflammatory pain, peripherally mediated and neuropathic pain, cough, lung edema, diarrhea, cardiac disorders, cardioprotection, depression, and cognitive, respiratory, diarrhea, irritable bowel syndrome and gastro-intestinal disorders, immunomodulation, and anti-tumor agents.
Owner:ALKERMES INC
Carboxylesterase inhibitors
This disclosure relates to amides, aryl sulphonamides, aryl ureas, and α,β-diketones derivatives useful as carboxylesterase esterase inhibitors. The disclosure is also directed to the use of these compounds as selective human intestinal carboxylesterase inhibitors and insect carboxylesterase inhibitors. The disclosure is also directed to pharmaceutical compositions and pesticide formulations containing these compounds, and to methods for treating or ameliorating the toxic effects following administration of drugs such as cancer therapy drugs, treating or ameliorating the effects of a drug overdose, and to the use of the compounds for increasing the effectiveness of insecticides and pesticides.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
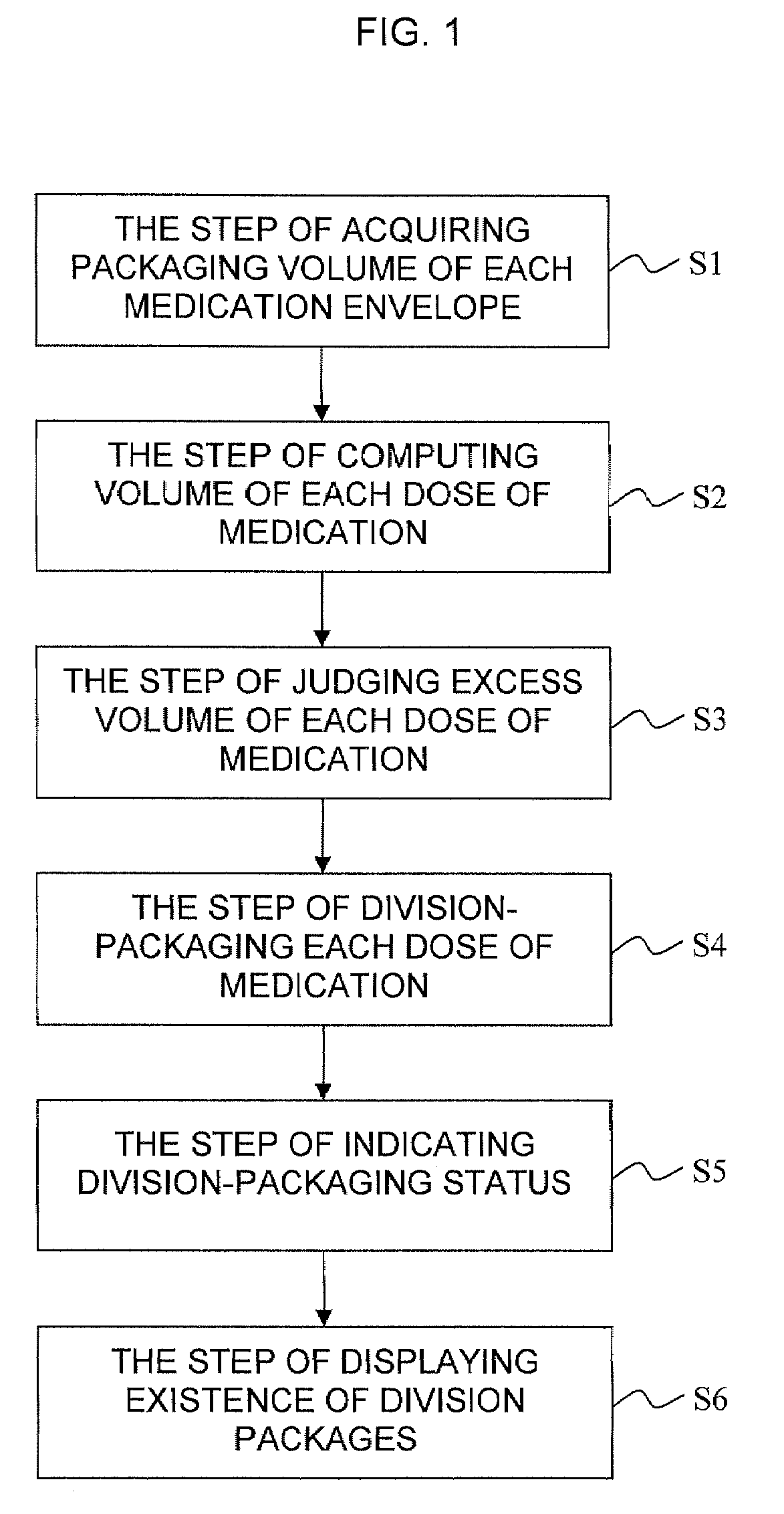
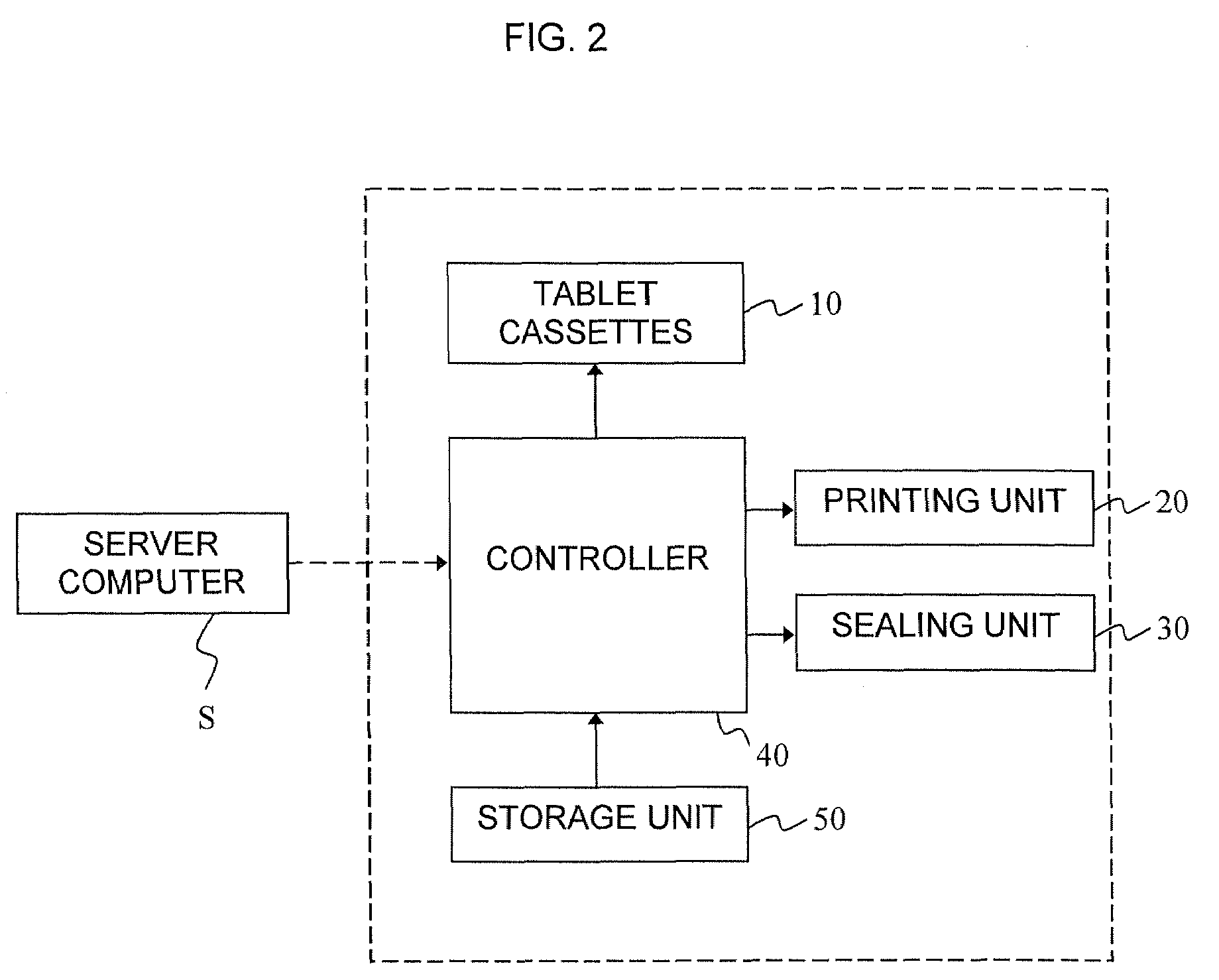
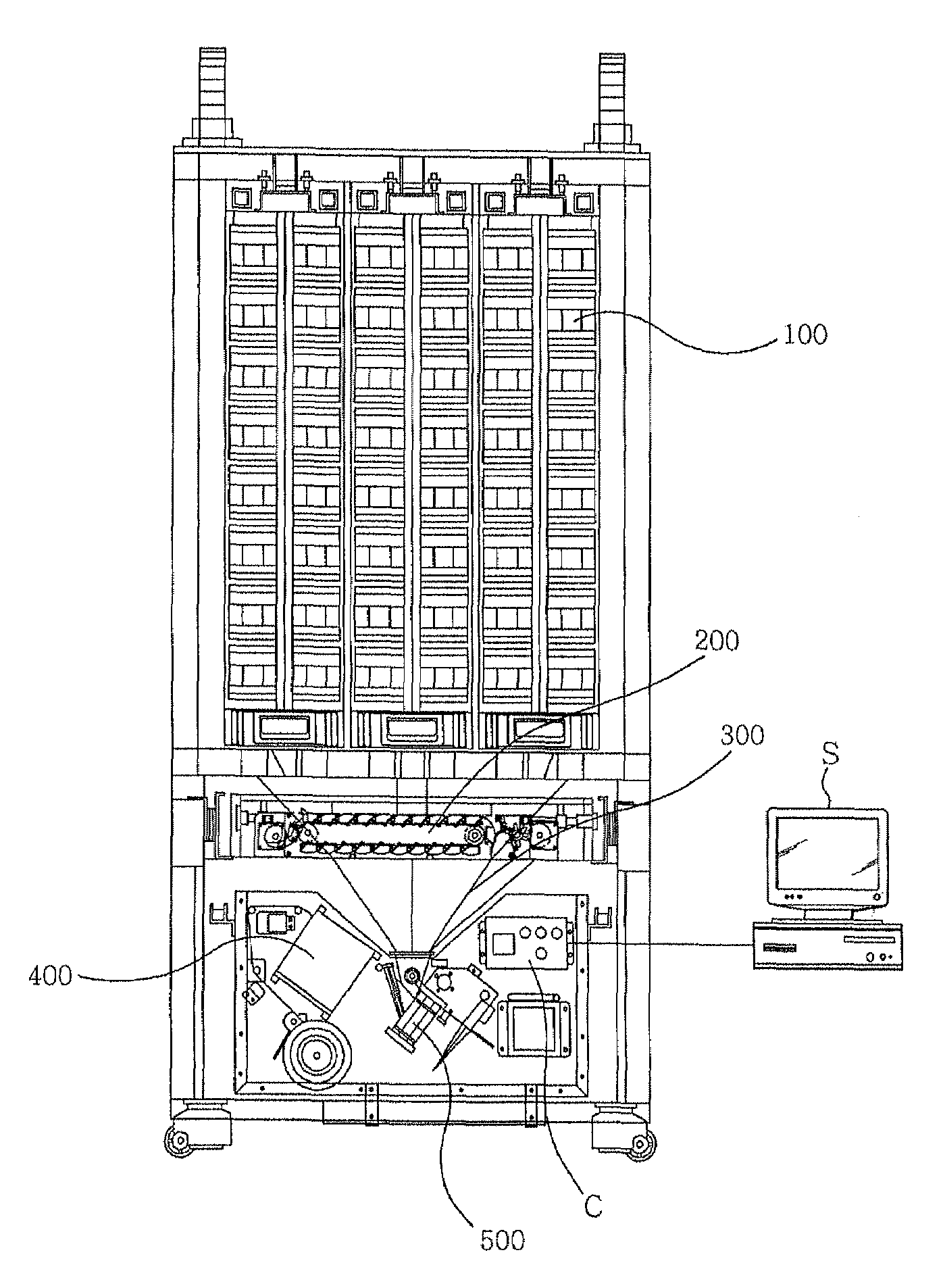
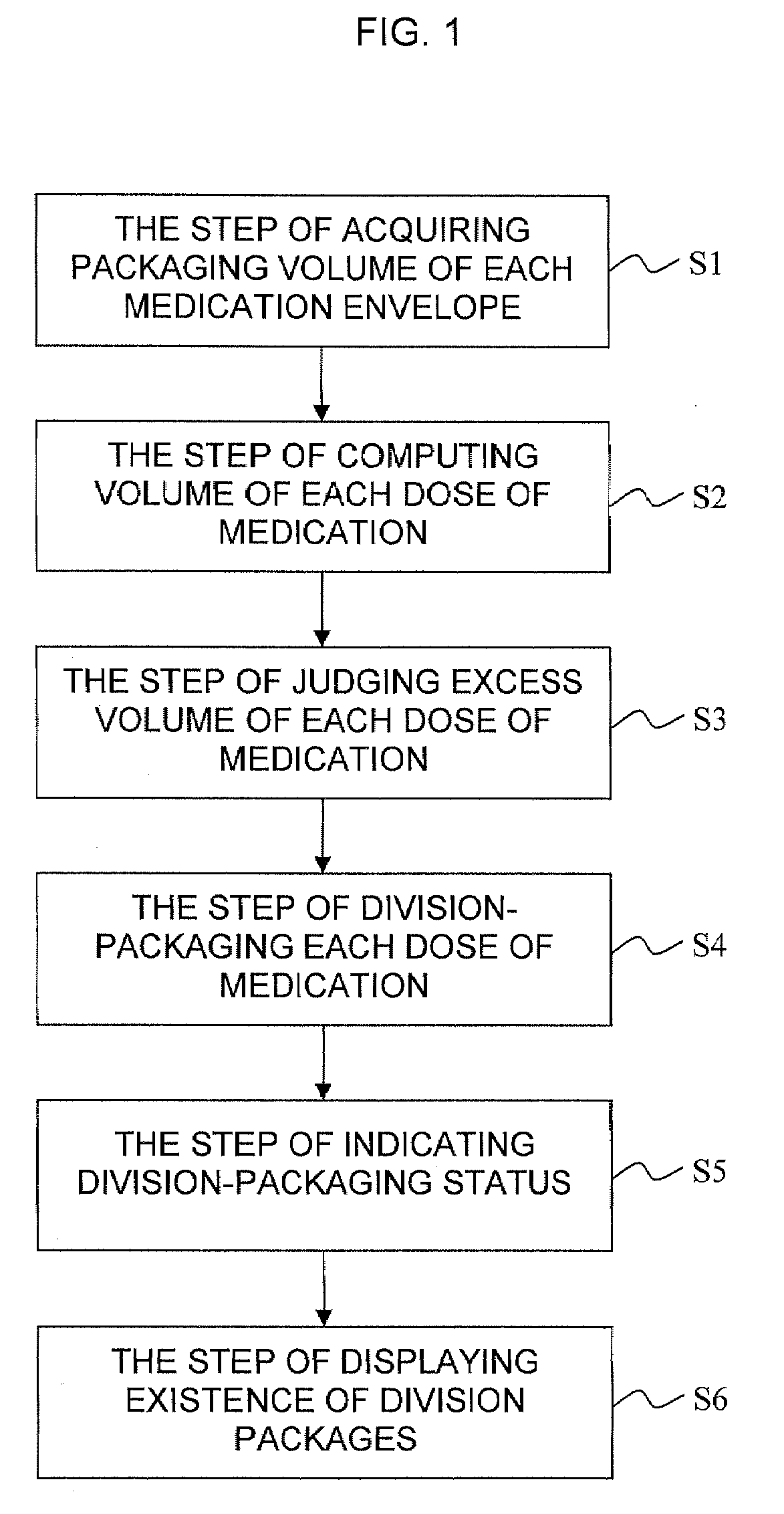
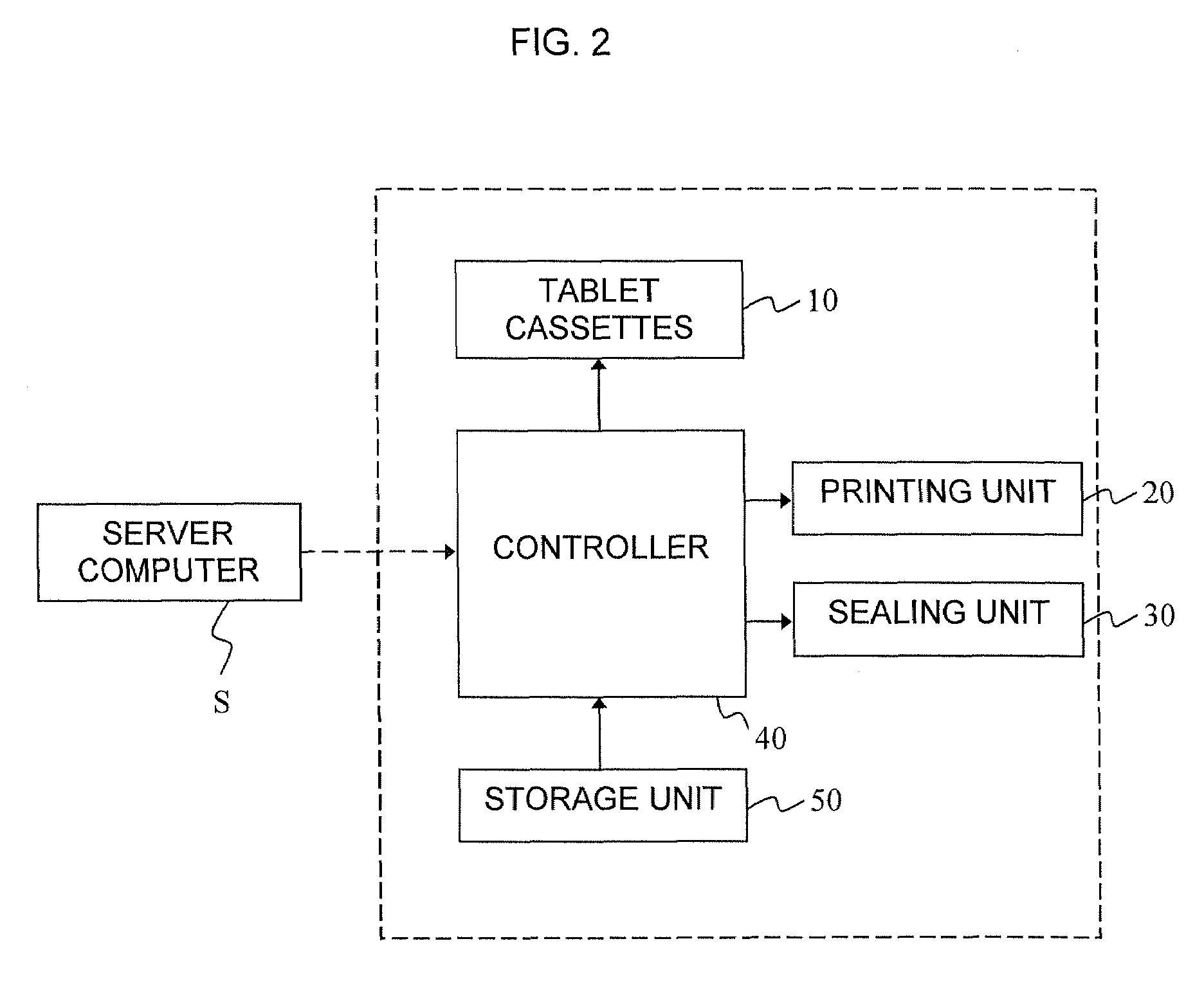
Division-packaging method and apparatus for automatic medicine packaging machine
ActiveUS7549268B2Prevent incapabilityFirm packagingSmall article dispensingOther accessoriesDrug overdoseDrug product
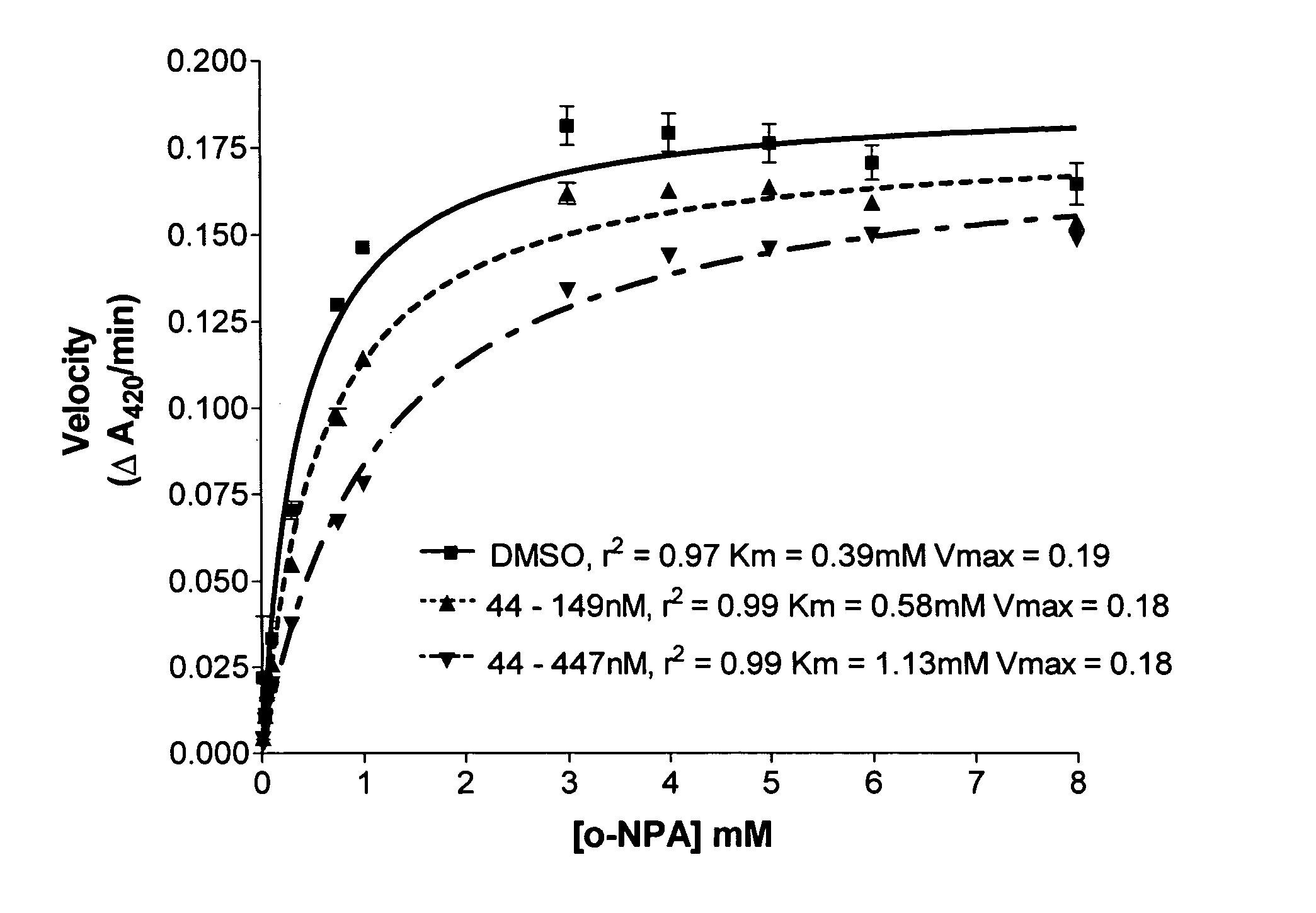
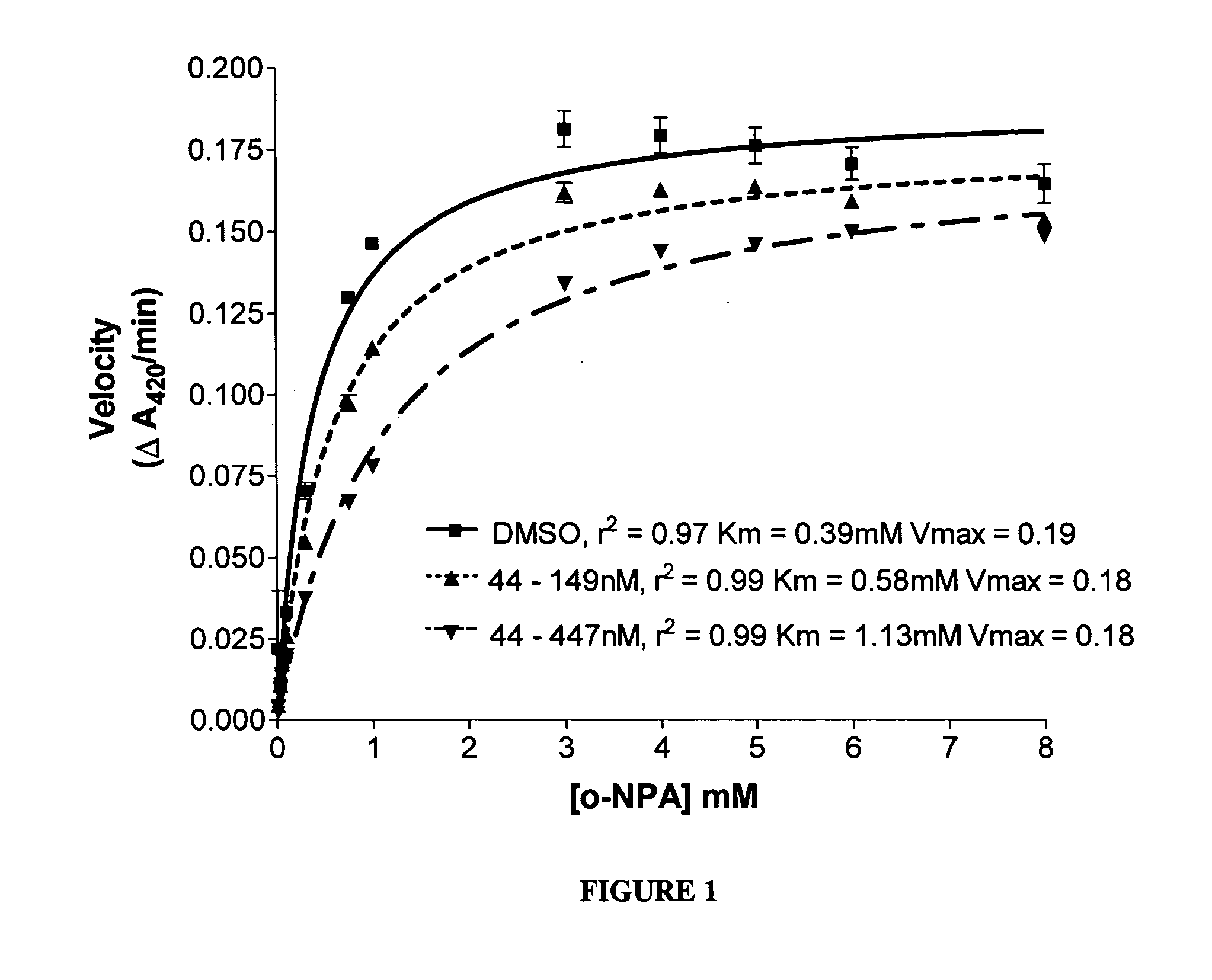
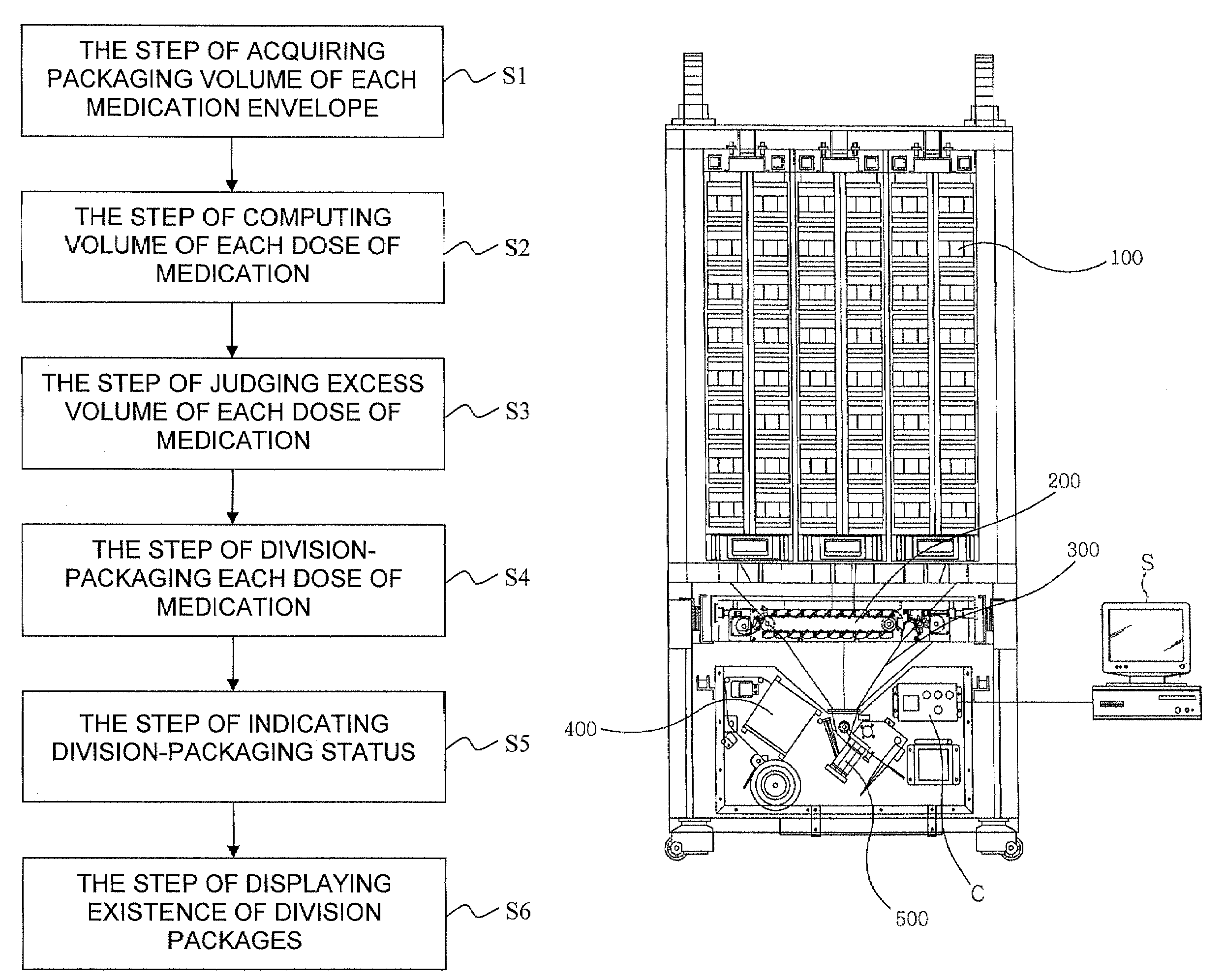
A division-packaging method for an automatic medicine packaging machine that allows stable packaging even when total volume of each dose of medication for making a package is bigger than packaging volume of any medication envelope established in the automatic medicine packaging machine and that prevents incapability of packaging due to excess volume of each dose of medication. The method includes step of acquiring packaging volume of each medication envelope; step of computing total volume of each dose of medication; step of judging excess volume of each dose of medication; and step of division-packaging each dose of medication when the total volume of each dose of medication for making a package is bigger than the packaging volume of any medication envelope established in the automatic medicine packaging machine.
Owner:JVM CO LTD
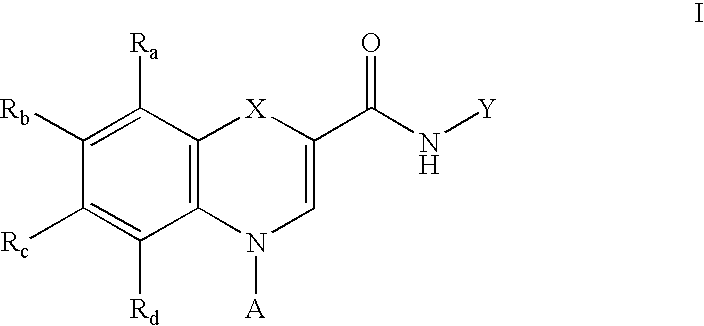
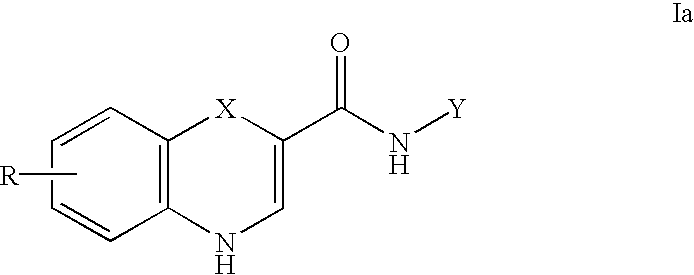
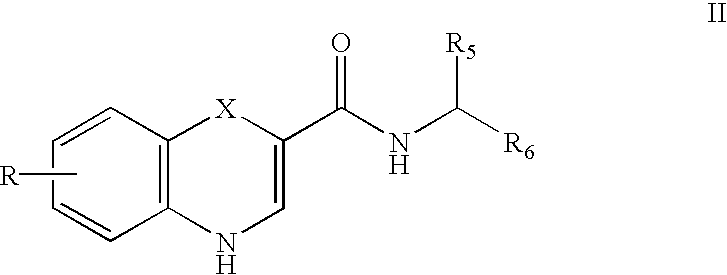
Substituted 4H-1,4-benzothiazine-2-carboxamide: GABA brain receptor ligands
Disclosed are 4H-1,4-Benzothiazine-2-carboxamides. These compounds are highly selective agonists, antagonists or inverse agonists for GABAa brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABAa brain receptors. These compounds are useful in the diagnosis and treatment of anxiety, depression, sleep, cognitive and seizure disorders, and overdose with benzodiazepine drugs and for enhancement of alertness. Pharmaceutical compositions, including packaged pharmaceutical compositions, are further provided. Compounds of the invention are also useful as probes for the localization of GABAA receptors in tissue samples.
Owner:NEUROGEN
Wearable narcosis alert
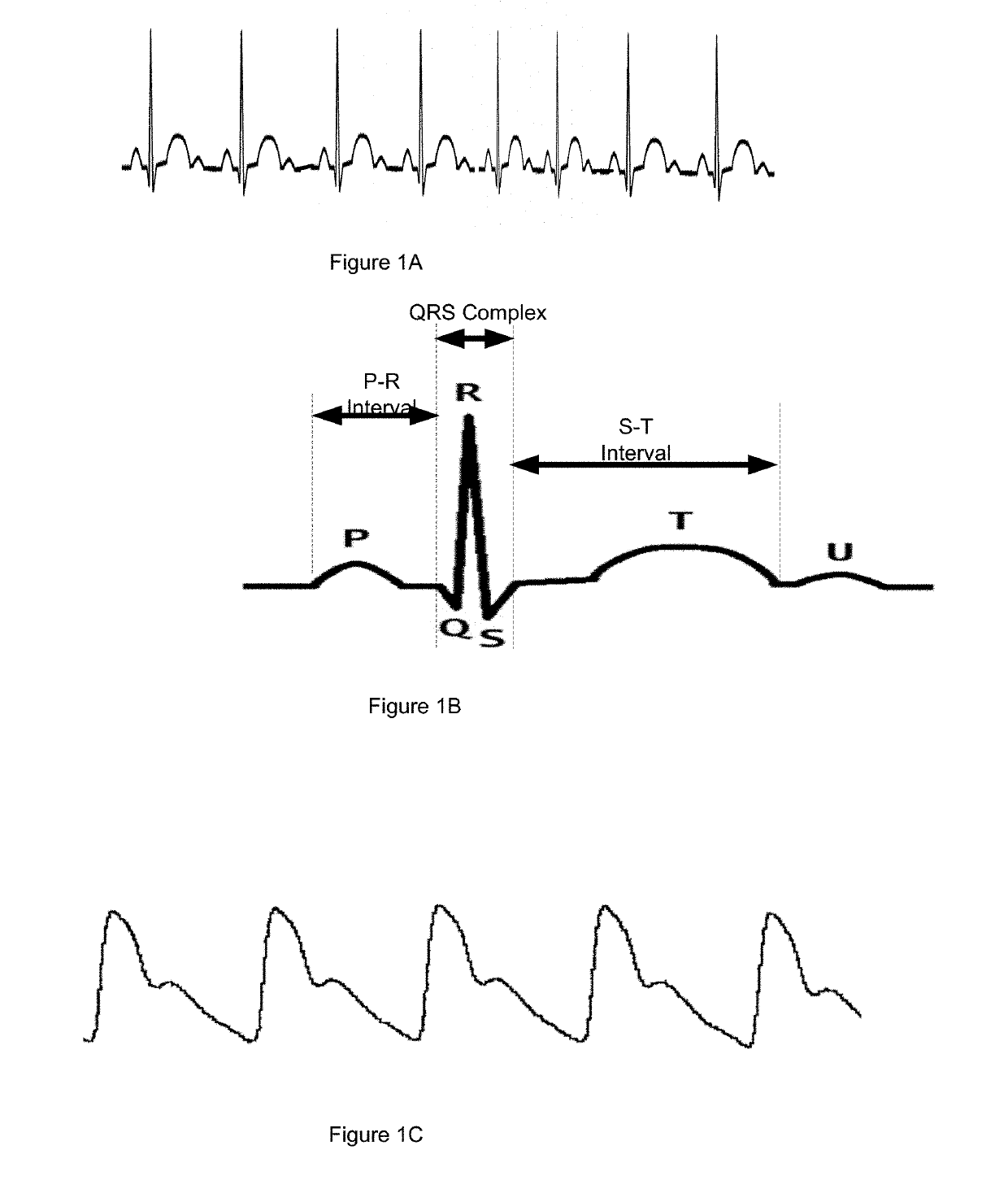
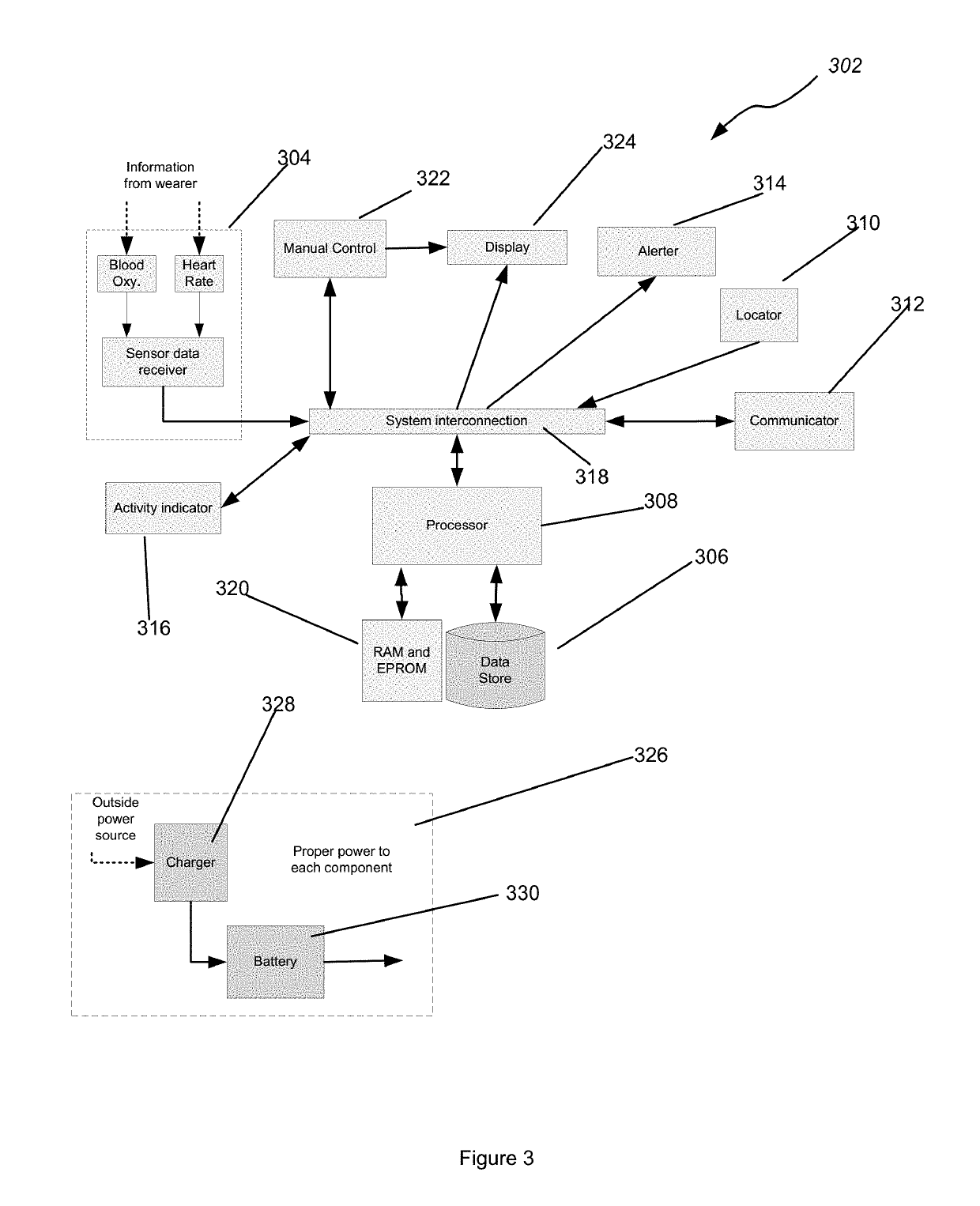
PendingUS20190209084A1Accurate and reliable processMedical communicationElectrocardiographySupporting systemTouch Perception
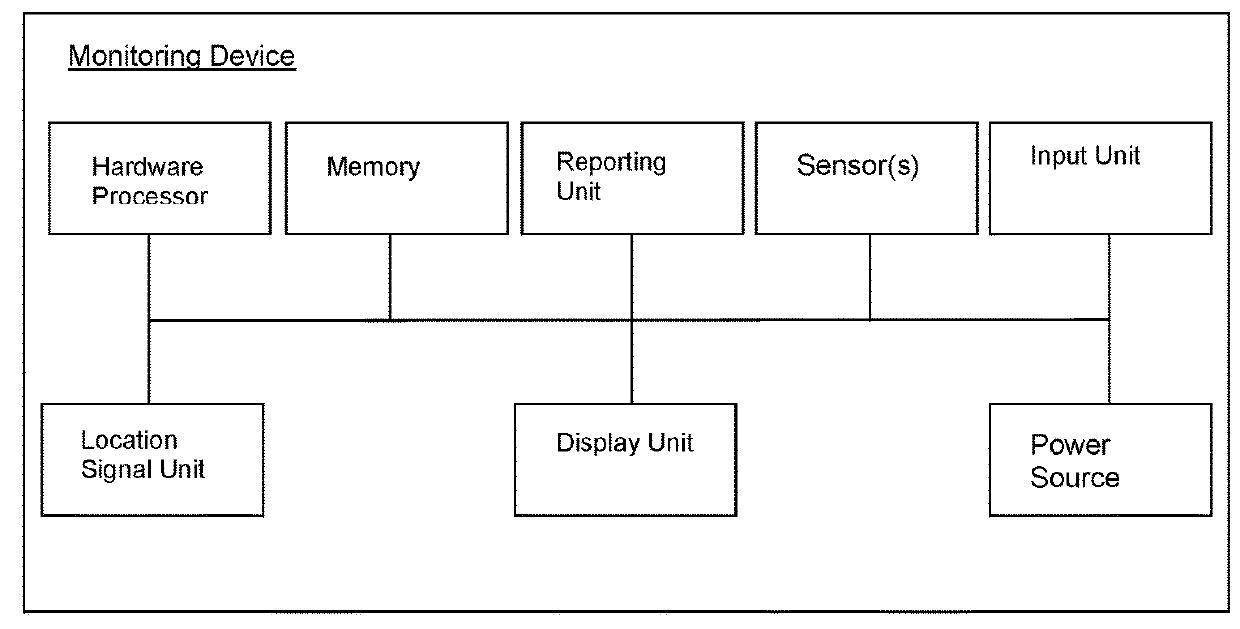
Apparatus worn by a person, methods, and support system for monitoring physiological parameters of the person, providing timely, actionable information to prevent death due to overdose, and, through social or professional influences, may cause life-saving changes in behavior. The apparatus sends alerts to the person via audible or tactile means, and to concerned others, that drug usage, drug overdose, or other critical medical condition has likely occurred. Alerts to concerned others include geographical information indicating where the person is, so that medical assistance personnel may find the person. Geographical information includes latitude, longitude, the translation of latitude and longitude into a street address, and elevation information indicating on which floor in a building the person is.
Owner:BRYANT V TYRONE +5
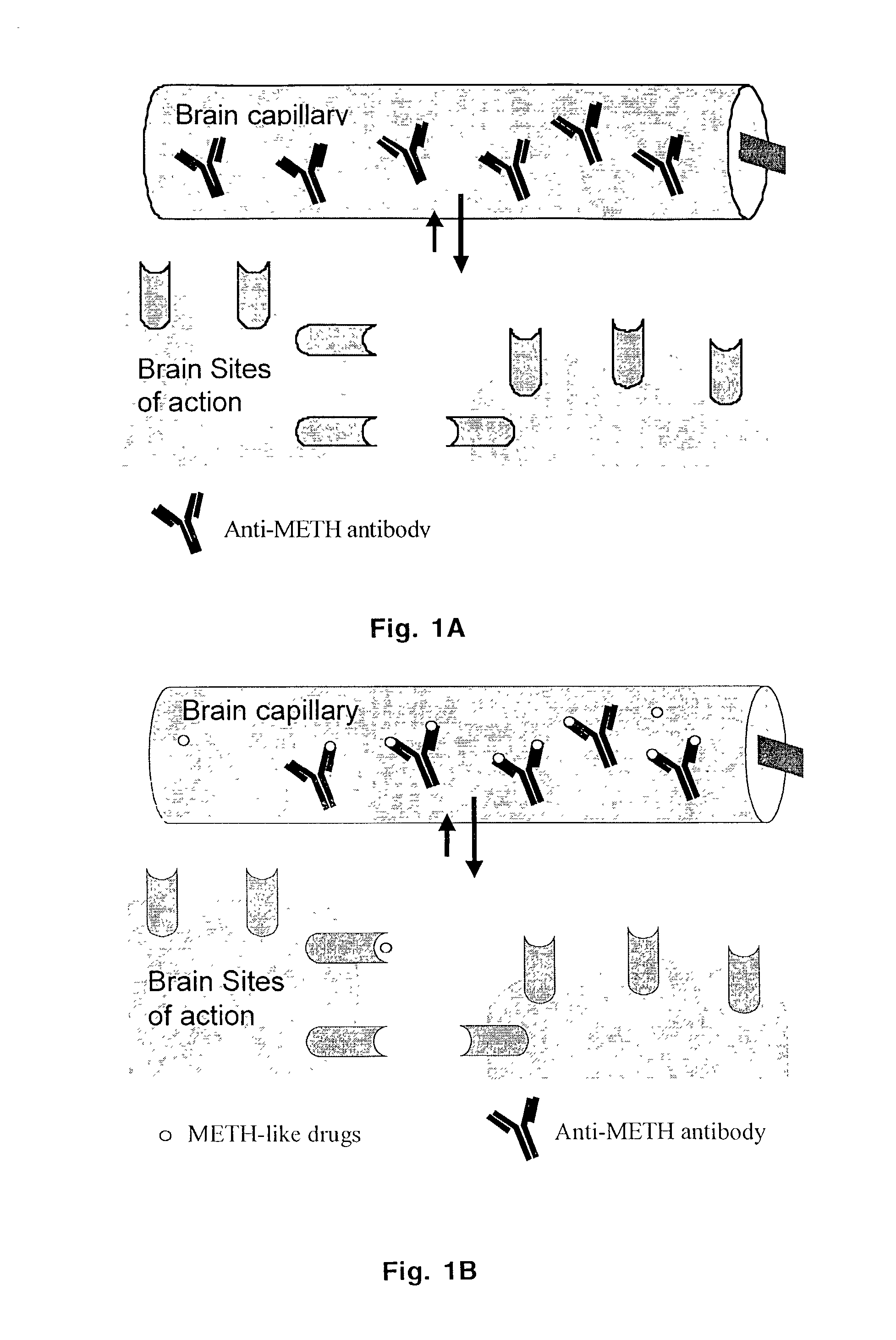
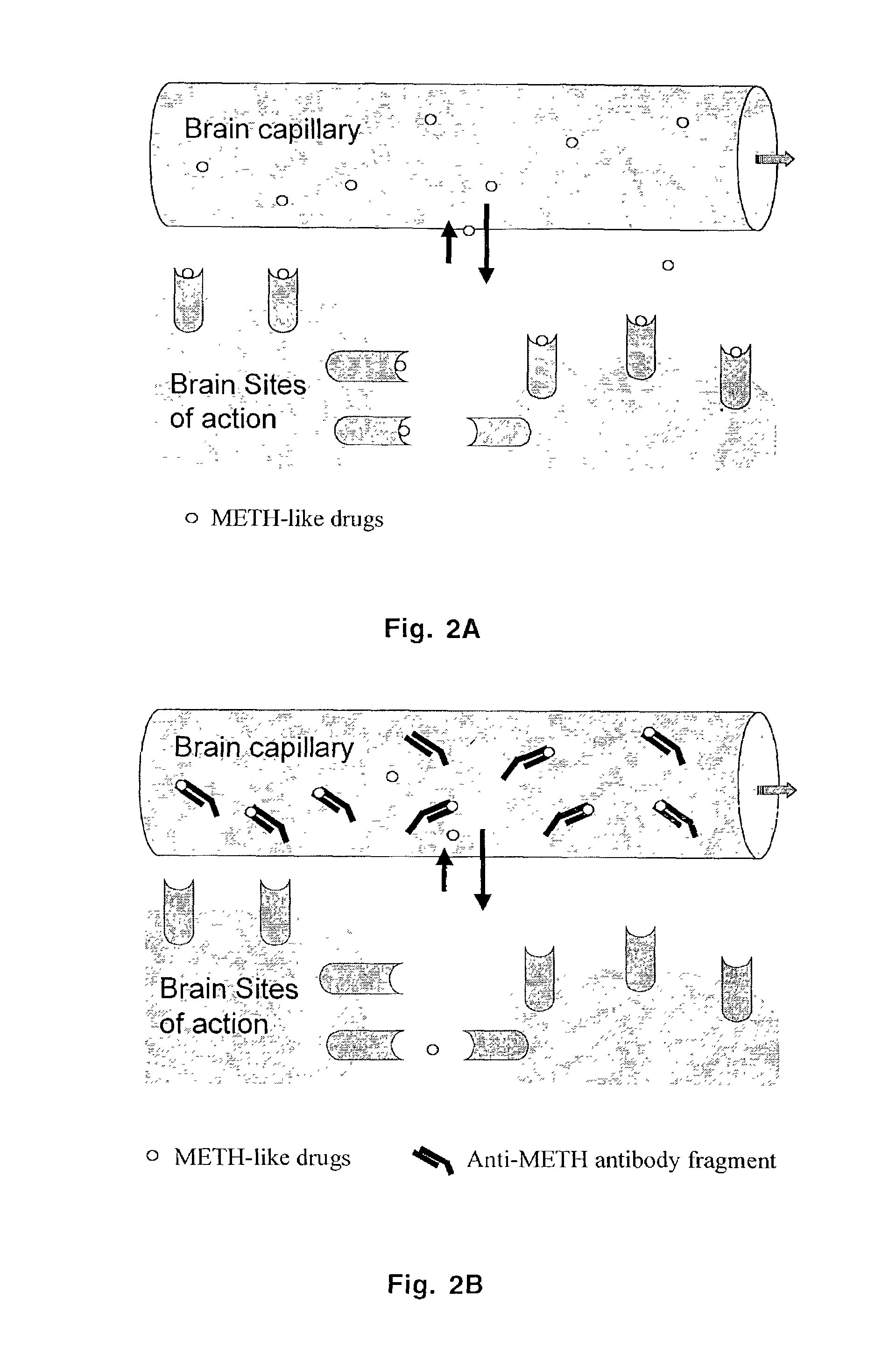
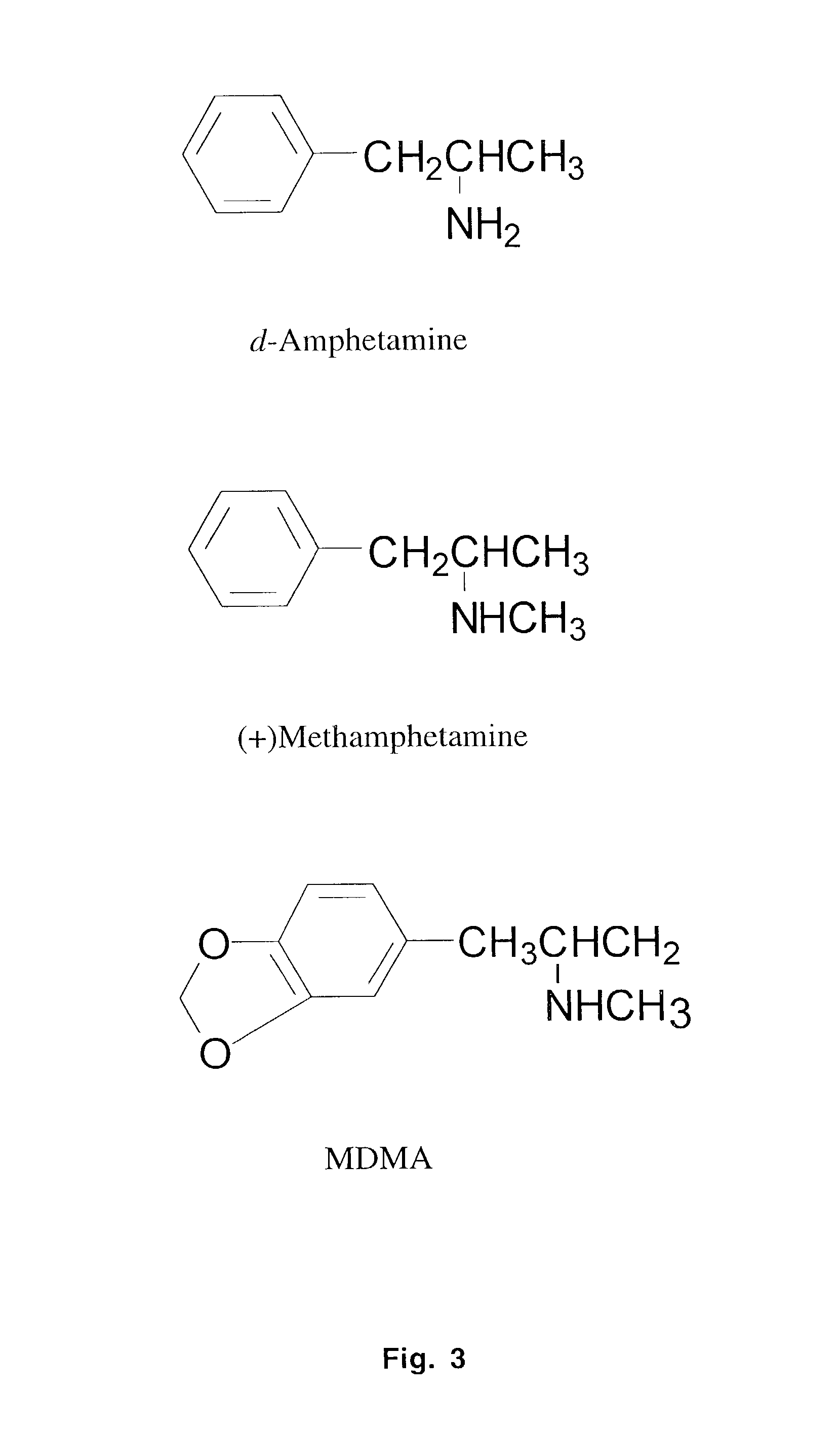
Monoclonal antibody antagonists for treating medical problems associated with d-amphetamine-like drugs
The present invention provides synthetic immunochemical haptens for the generation of antibodies that are designed to recognize the common molecular features of d-methamphetamine-like abused stimulants with insignificant cross-reactivity to endogenous substrates (e.g. dopamine) or over-the-counter medications (e.g. 1-methamphetamine, pseudoephedrine, phenylpropanolamine and ephedrine). These monoclonal antibodies and their antigen binding fragments are useful in treatment plans for recovering addicts, in emergency room settings for rapidly reversing a drug overdose, in protection of fetuses from drug-abusing pregnant mothers or in a psychiatric setting to reduce the exacerbation of psychotic disorders caused by stimulant drugs.
Owner:ARKANSAS FOR MEDICAL SCI THE UNIV OF +1
Division-packaging method and apparatus for automatic medicine packaging machine
ActiveUS20080149522A1Firm packagingPrevent incapabilitySmall article dispensingOther accessoriesDrug overdoseDrug product
Disclosed is a division-packaging method for an automatic medicine packaging machine that allows stable packaging even when total volume of each dose of medication for making a package is bigger than packaging volume of any medication envelope established in the automatic medicine packaging machine and that prevents incapability of packaging due to excess volume of each dose of medication. The method includes step of acquiring packaging volume of each medication envelope; step of computing total volume of each dose of medication; step of judging excess volume of each dose of medication; and step of division-packaging each dose of medication when the total volume of each dose of medication for making a package is bigger than the packaging volume of any medication envelope established in the automatic medicine packaging machine.
Owner:JVM CO LTD
Emetic embedded capsule
This invention provides capsules containing an emetic, which capsules can encapsulate a drug, wherein the amount of emetic and the amount of drug is such that the number of capsules needed to be ingested to cause emesis is fewer than the number of capsules needed to be ingested to cause overdose of the drug, so that if a person takes an overdose of the emetic encapsulated drug, he or she will vomit before the drug is absorbed by the body.
Owner:PROXIMATE CONCEPTS LLC
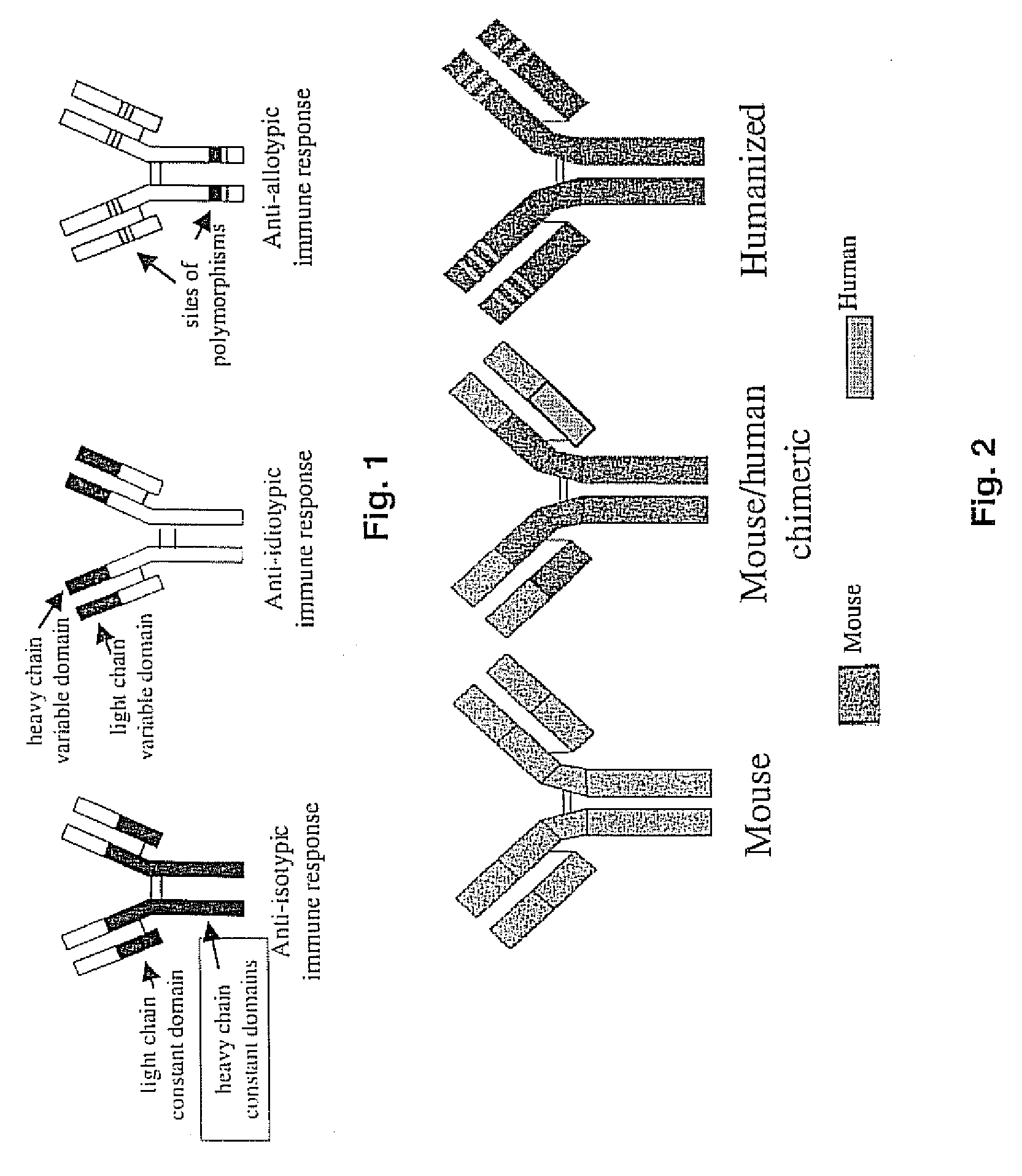
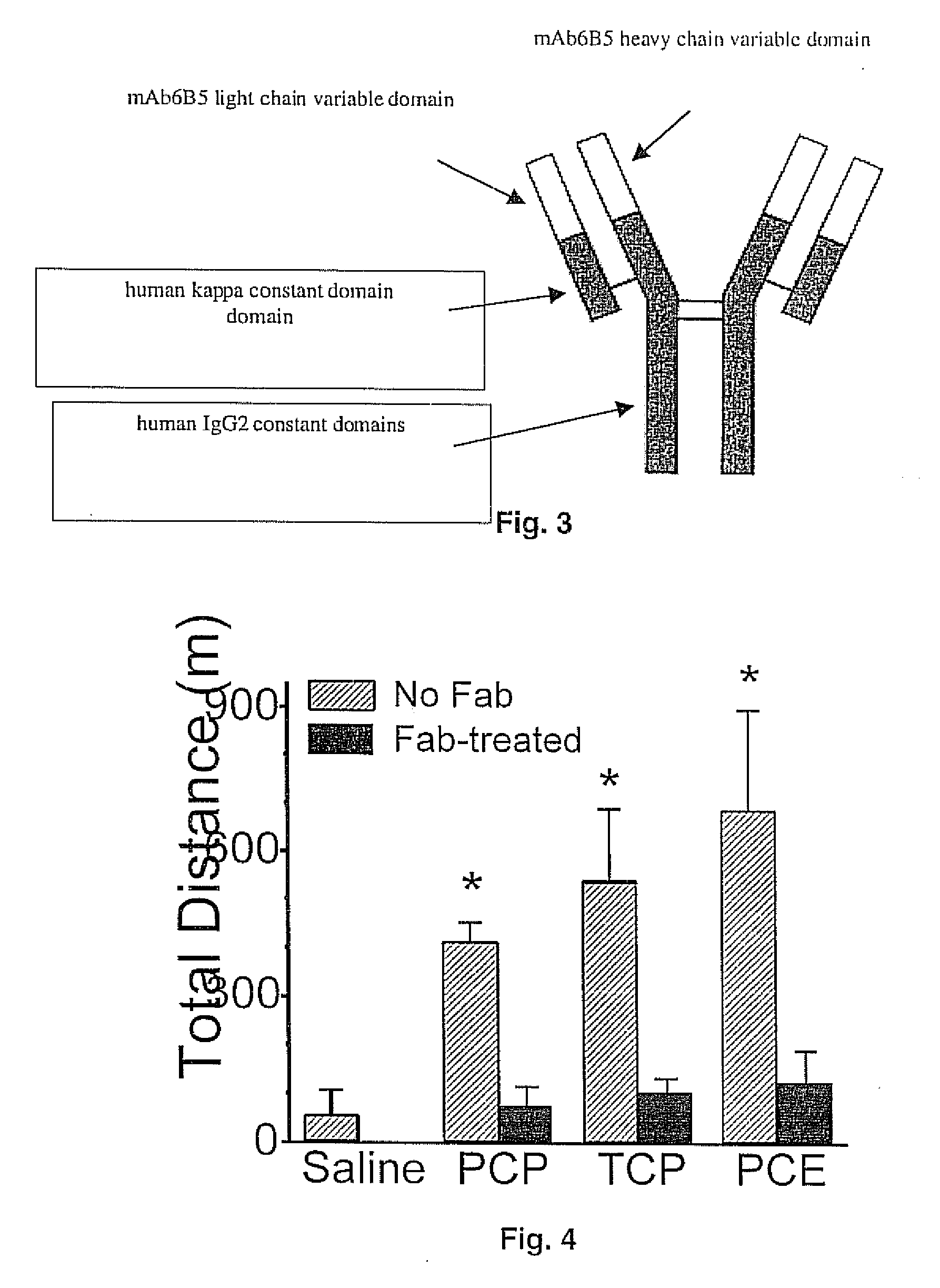
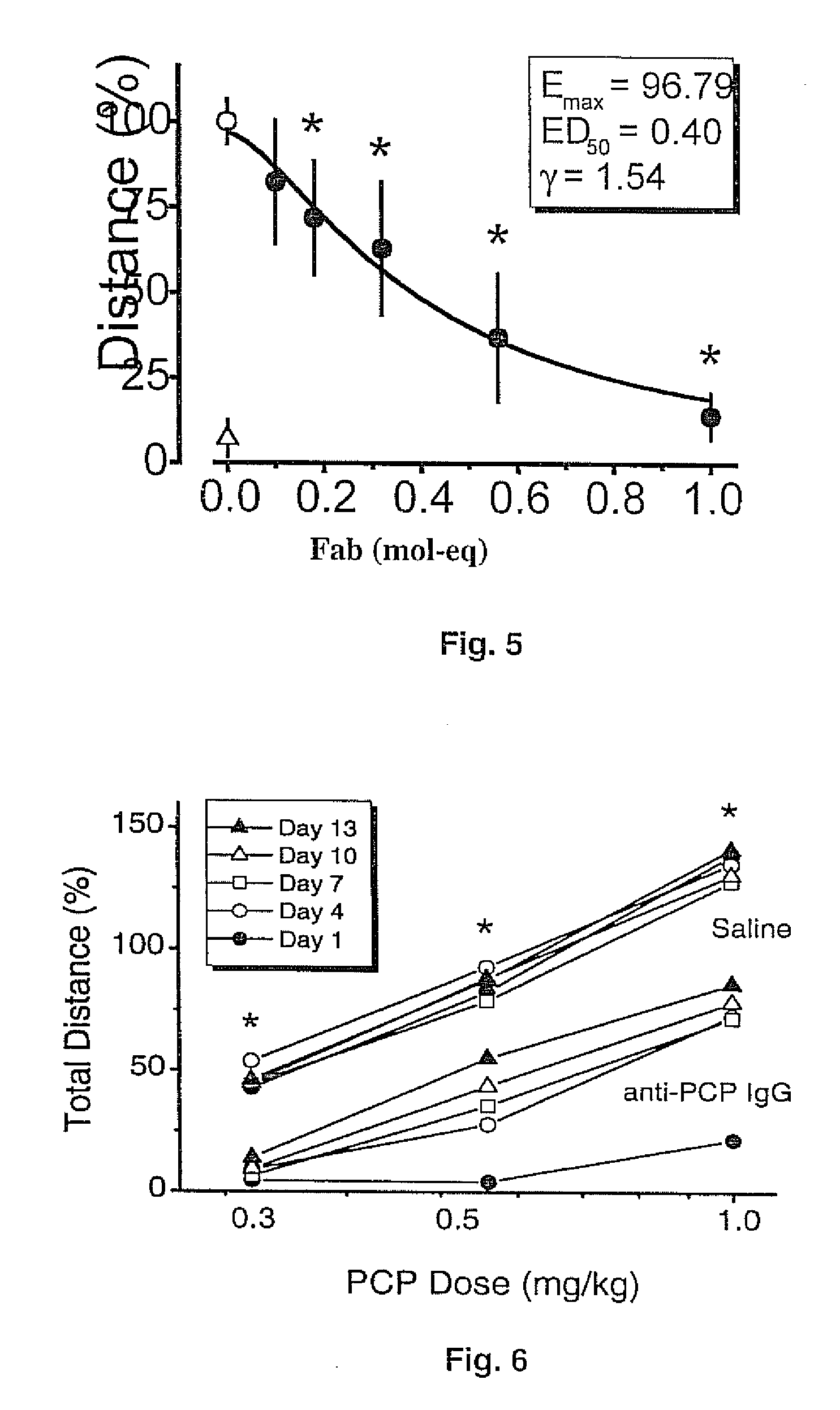
Mouse/human chimeric Anti-phencyclidine antibody and uses thereof
InactiveUS20070207145A1Safe and effectiveLow immunogenicityAntibody ingredientsImmunoglobulinsMortality rateImmunogenicity
The present invention provides a chimeric mouse / human antibody (ch-mAb6B5) for treatment of abuse and toxicity of the arylcyclohexylamines class of drugs (i.e., phencyclidine- or PCP-like drugs). This antibody comprises light and heavy chain PCP binding regions of mouse mAb6B5, coupled to the light and heavy chain constant regions of a human kappa IgG2 or IgG4 isoform. Also provided are the DNA and amino acid sequences of the chimeric light and heavy chain of this antibody. Further provided are data that demonstrate that the new chimeric antibody retains the high affinity and specificity of a previously generated mouse anti-PCP monoclonal antibody (mAb6B5) yet being minimally immunogenic since it has human immunoglobulin constant region. This new medication would allow safe and effective treatment of PCP drug overdose, decrease mortality, and reduce harmful effects due to excessive and prolonged PCP drug use.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Polycyclic amines as opioid receptor modulators
ActiveUS20180258065A1Group 3/13 element organic compoundsMagnesiaAlcohol addictionNeurological disorder
The present invention provides a genus of polycyclic amines that are useful as opioid receptor modulators. The compounds of the invention are useful in both therapeutic and diagnostic methods, including for treating pain, neurological disorders, cardiac disorders, bowel disorders, drug and alcohol addiction, drug overdose, urinary disorders, respiratory disorders, sexual dysfunction, psoriasis, graft rejection or cancer.
Owner:ECSTASY LLC
Substituted fused pyrroleoximes and fused pyrazoleoximes
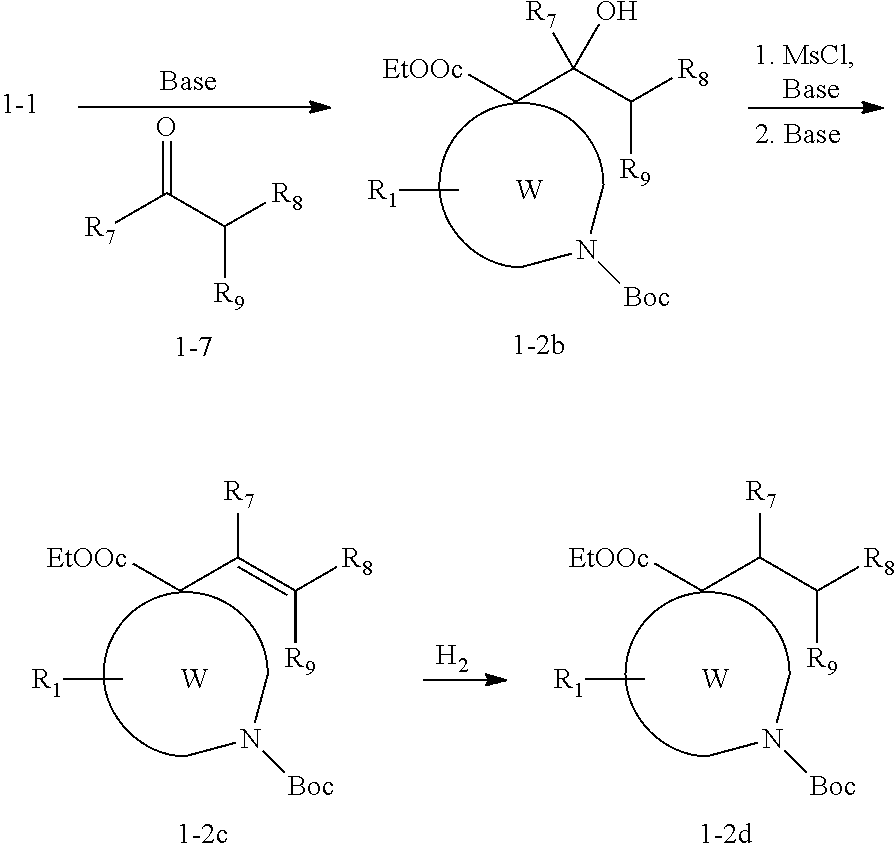
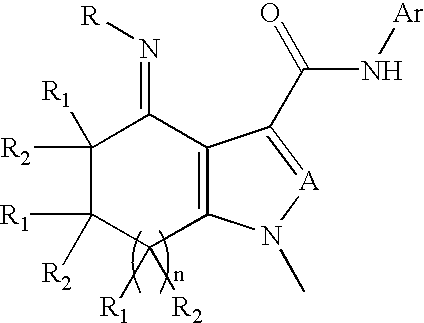
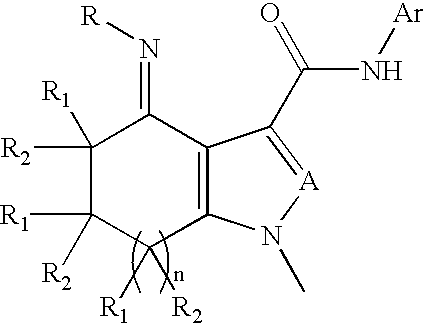
InactiveUS20020128236A1High affinityHigh selectivityBiocideNervous disorderBenzodiazepineDrug overdose
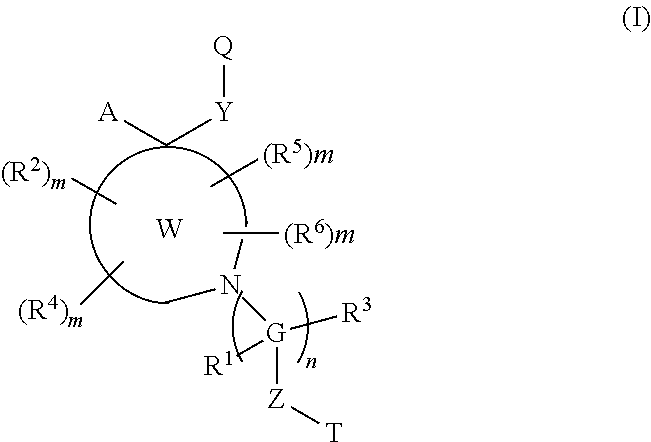
Disclosed are compounds of the formula 1 and the pharmaceutically acceptable salts thereof wherein R, Ar, A, n, R.sub.1 and R.sub.2 are defined herein. These compounds are highly selective agonists, antagonists or inverse agonists for GABA.sub.A brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABA.sub.A brain receptors and are therefore useful in the diagnosis and treatment of anxiety, depression, Down Syndrome, sleep and seizure disorders, overdose with benzodiazepine drugs and for enhancement of memory. Pharmaceutical compositions, including packaged pharmaceutical compositions, are also disclosed.
Owner:NEUROGEN
AMIDE, ARYL SULFONAMIDE, ARYL UREA, AND a,b-DIKETONE DERIVED CARBOXYLESTERASE INHIBITORS, AND THEIR METHODS OF USE
InactiveUS20080146548A1Improve efficiencyBiocideAmide active ingredientsKetoneIntestinal Carboxylesterase
This disclosure relates to amides, aryl sulphonamides, aryl ureas, and α,β-diketones derivatives useful as carboxylesterase esterase inhibitors. The disclosure is also directed to the use of these compounds as selective human intestinal carboxylesterase inhibitors and insect carboxylesterase inhibitors. The disclosure is also directed to pharmaceutical compositions and pesticide formulations containing these compounds, and to methods for treating or ameliorating the toxic effects following administration of drugs such as cancer therapy drugs, treating or ameliorating the effects of a drug overdose, and to the use of the compounds for increasing the effectiveness of insecticides and pesticides.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
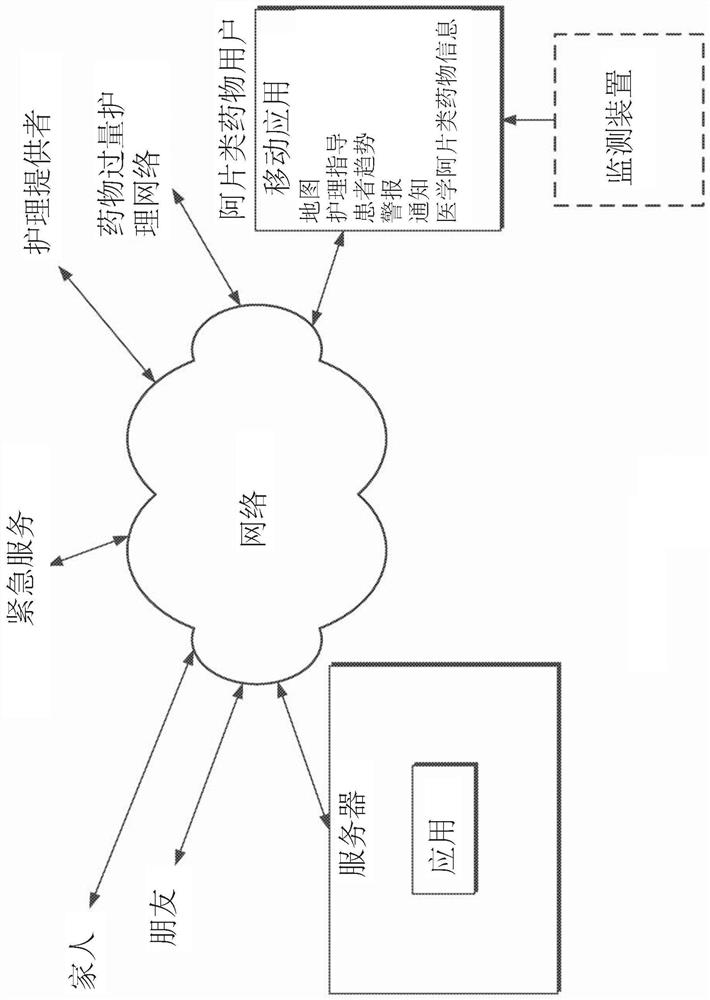
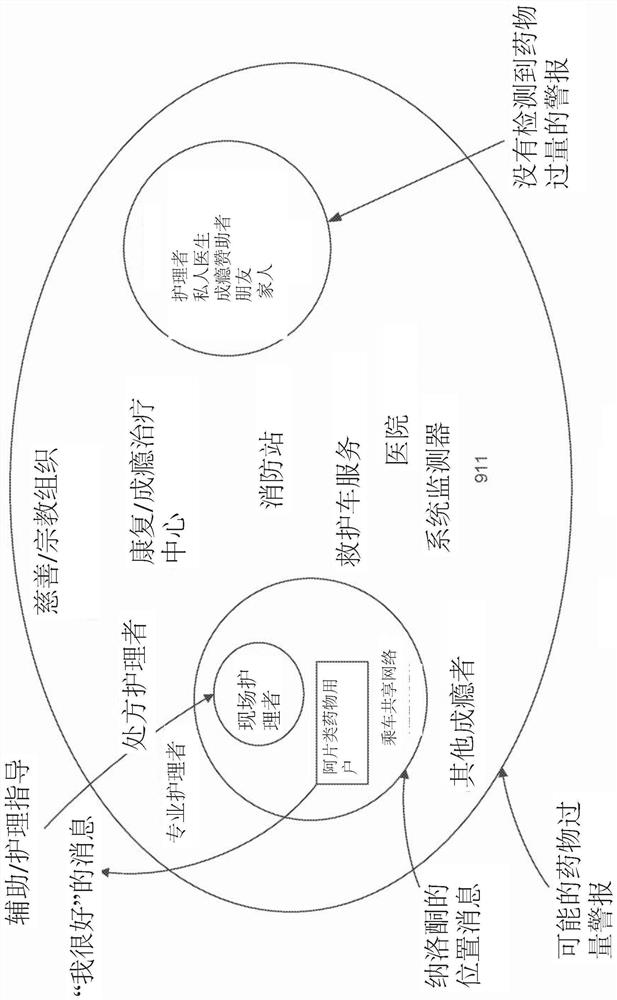
Device and methods for monitoring or preventing misuse or abuse of analgesics
The invention relates to methods and devices for measuring or monitoring a subject undergoing one or more therapeutic treatments in real time to prevent drug abuse or misuse. The devices of the invention are intended to be worn or carried by the subjects, and they can thus be defined as portable or wearable devices. The devices are further configured to monitor the subjects and to prevent potential drug abuse or drug overdose.
Owner:ISVIAL LLC
Using method of flowing speed adjusting device for nursing
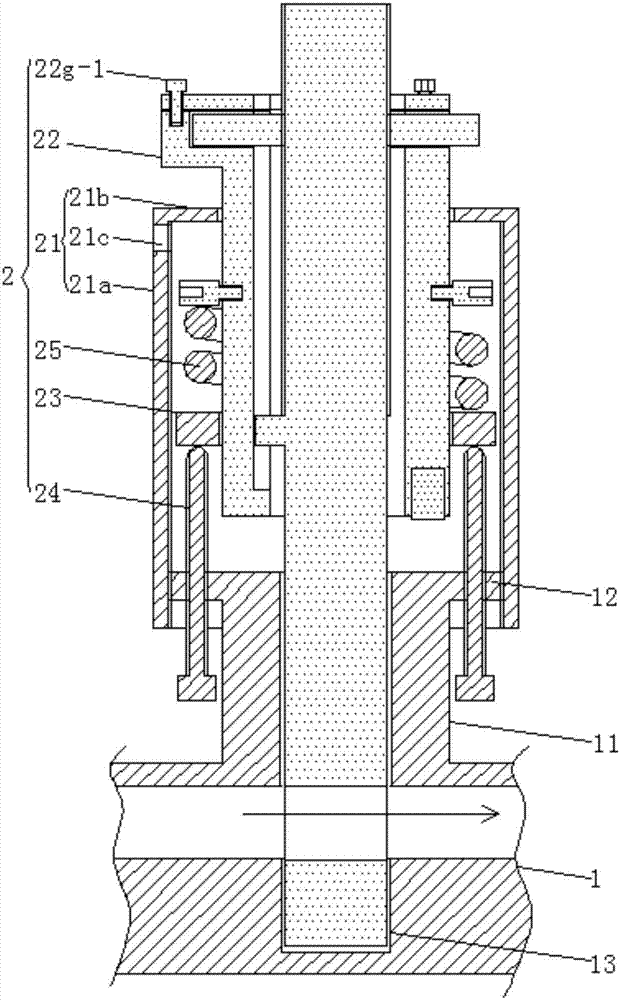
InactiveCN107335109AReasonable structureEase of mass productionMedical devicesFlow monitorsDrug overdoseNormal rate
The invention relates to a method for using a flow rate regulating device for nursing. The liquid flow rate regulating device is used to regulate the flow rate. The liquid flow rate regulating device includes an infusion tube and a side regulating device; the regulating method includes the following steps: Step 1, assembling : Step 1.1, assembling the speed regulating plunger; Step 1.2, placing the adjusting bolt, adjusting washer, and compression spring; Step 1.3, assembling the upper limit piece; Step 2. Infusion; Step 3. Adjusting the flow rate; Step 4. Give the drug quickly. The liquid flow rate regulating device used in the present invention has a reasonable structure, is convenient for mass production, and is convenient and simple to assemble; the method of the present invention can adjust the rate of infusion under normal conditions; the method can also be used in emergency situations. Adjusting the pressing damping of the speed-regulating plunger can further close the rapid drug delivery function; this method can further prevent long-term rapid drug delivery by delaying the alarm, causing drug overdose.
Owner:刘昕烨
Heteroaryl fused aminoalkyl imidazole derivates: selective modulators of GABAA receptors
Disclosed are compounds of the formula: or the pharmaceutically acceptable non-toxic salts thereof wherein the A, B, C, D, X, R1, R2, R3, R4, R5, and R6, are variables defined herein, which compounds are highly selective agonists, antagonists or inverse agonists for GABAa brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABAa brain receptors, and are therefore useful in the diagnosis and treatment of anxiety, Down Syndrome, sleep, cognitive and seizure disorders, depression, overdose with benzodiazepine drugs, and enhancement of memory and alertness.
Owner:NEUROGEN
Polycyclic amines as opioid receptor modulators
ActiveUS10676456B2Group 3/13 element organic compoundsMagnesiaAlcohol addictionNeurological disorder
The present invention provides a genus of polycyclic amines that are useful as opioid receptor modulators. The compounds of the invention are useful in both therapeutic and diagnostic methods, including for treating pain, neurological disorders, cardiac disorders, bowel disorders, drug and alcohol addiction, drug overdose, urinary disorders, respiratory disorders, sexual dysfunction, psoriasis, graft rejection or cancer.
Owner:ECSTASY LLC
Device and methods for monitoring or preventing misuse or abuse of analgesics
The invention relates to methods and devices for measuring or monitoring a subject undergoing one or more therapeutic treatments in real time to prevent drug abuse or misuse. The devices of the invention are intended to be worn or carried by the subjects, and they can thus be defined as portable or wearable devices. The devices are further configured to monitor the subjects and to prevent potential drug abuse or drug overdose.
Owner:ISVIAL LLC
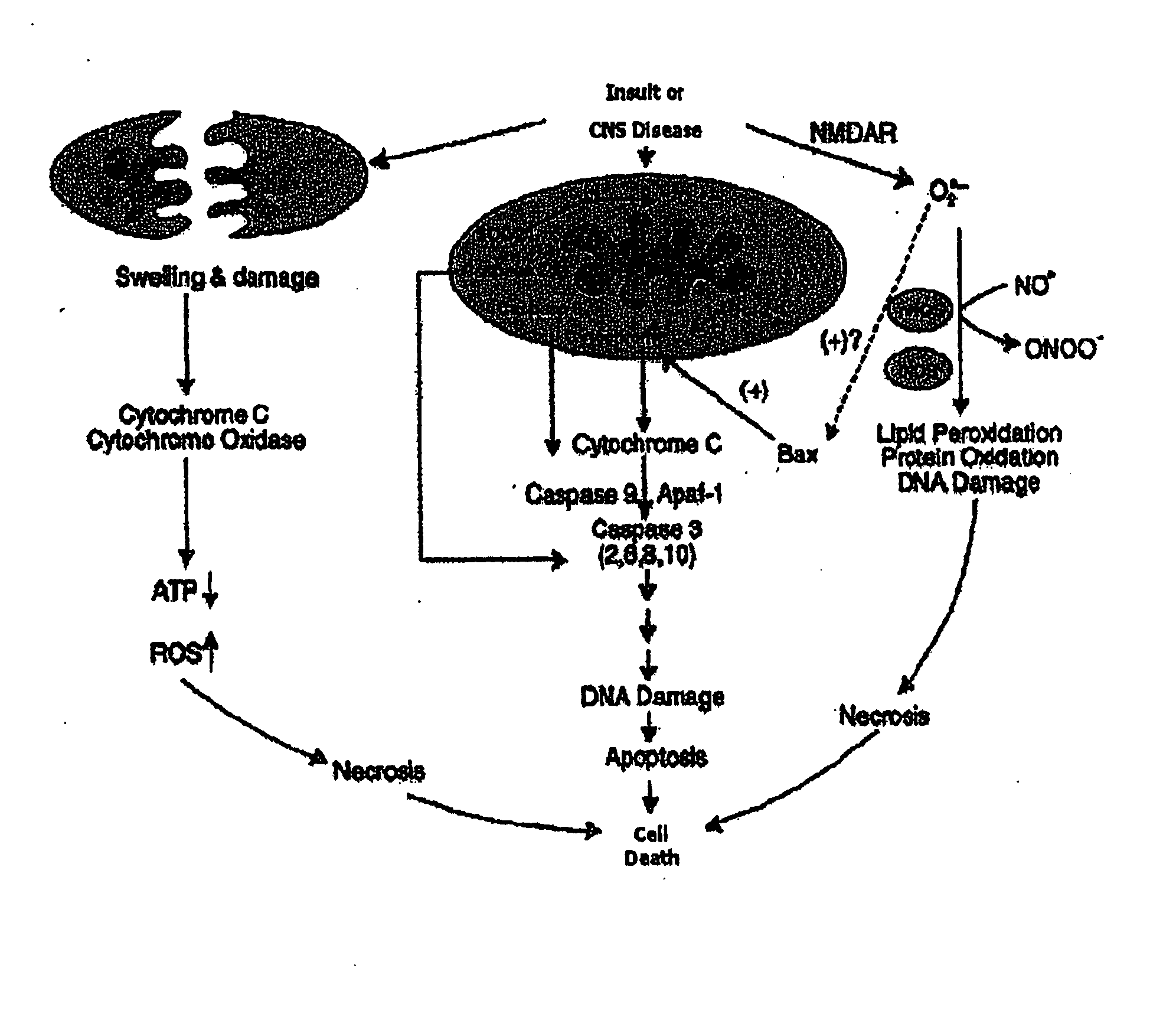
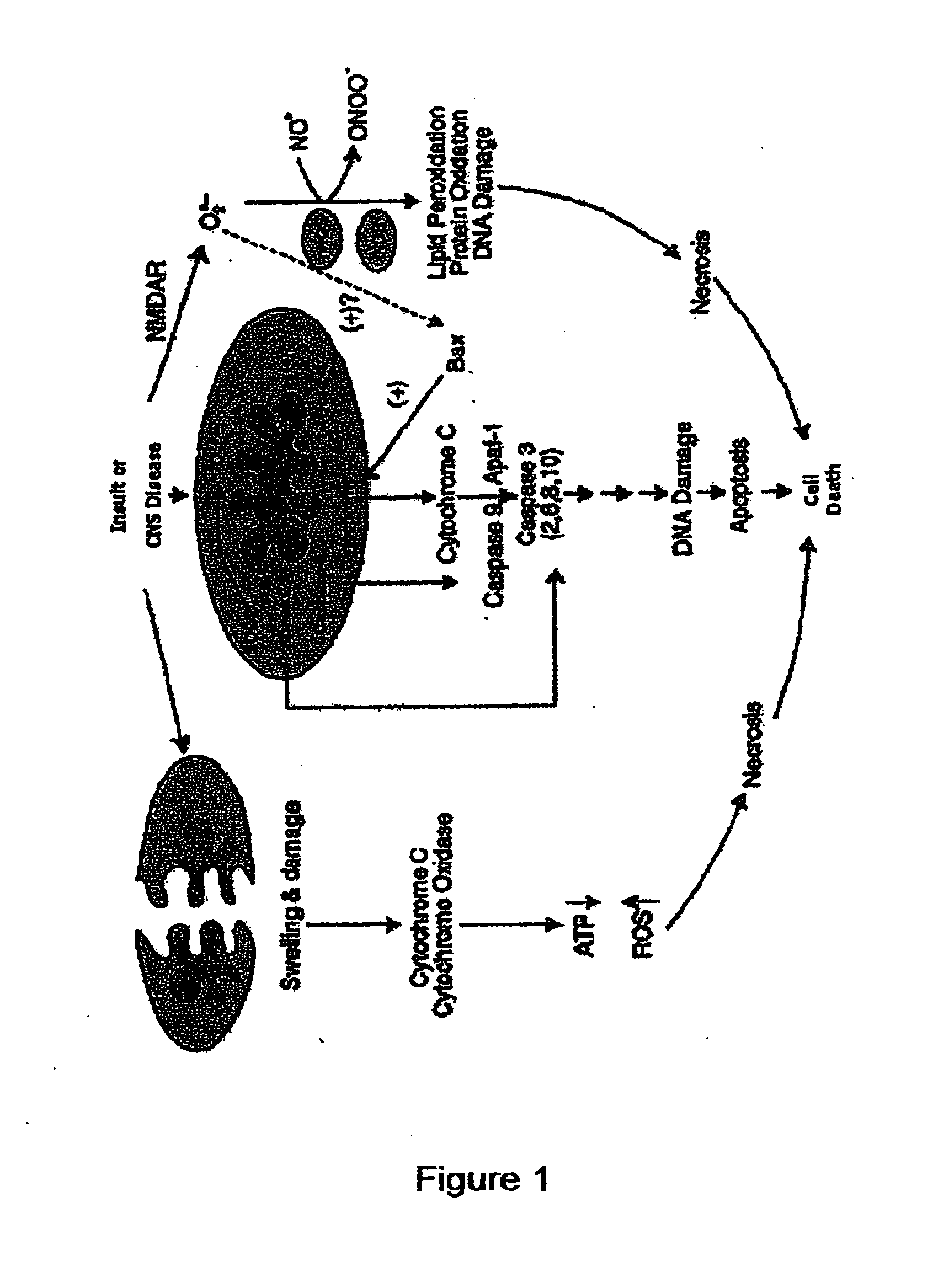
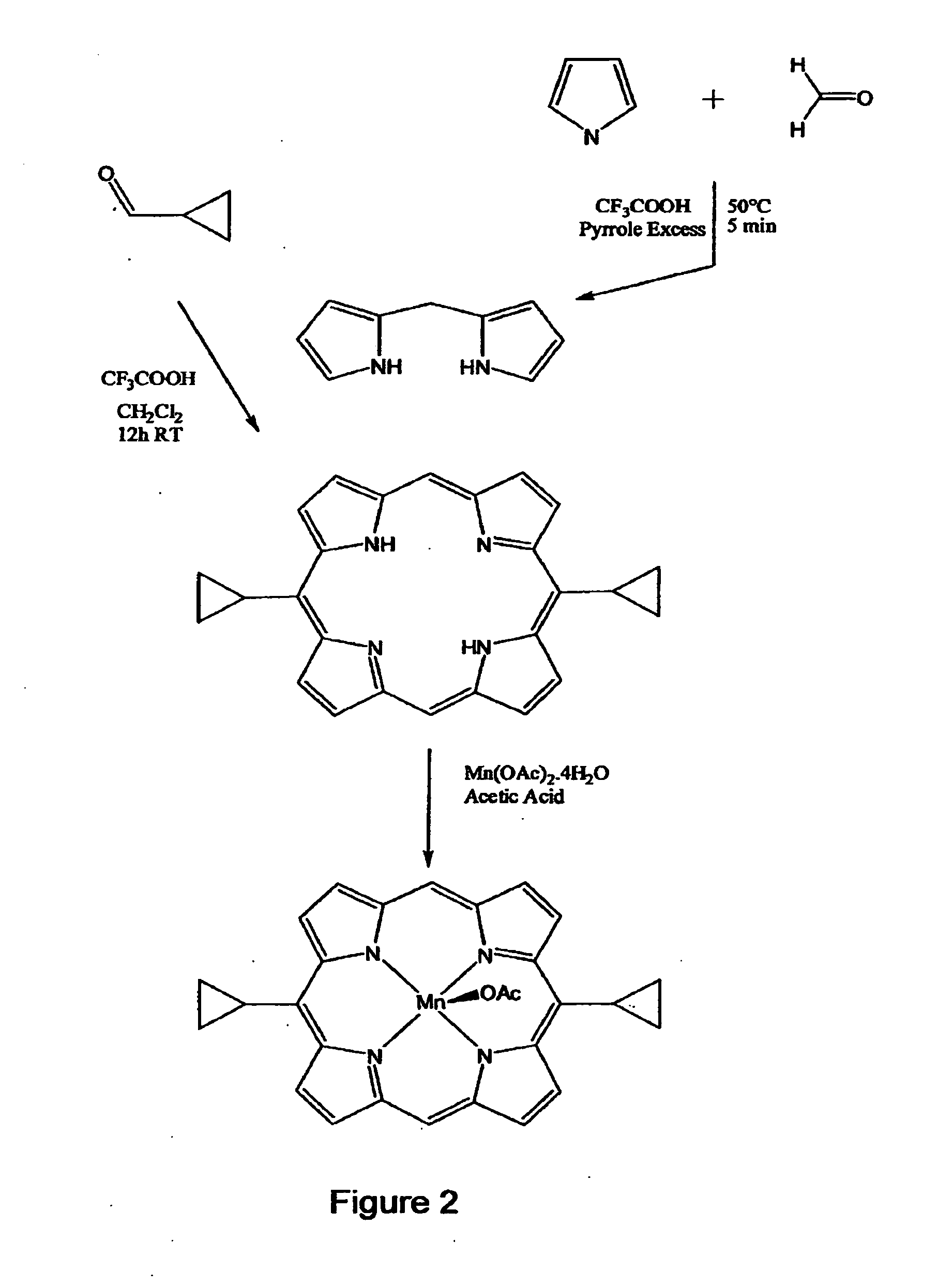
Anti-apoptotic benzodiazepine receptor ligand inhibitors
InactiveUS20100120738A1Inhibit delay prevent bindingSufficient stability and solubility and oral bioavailabilityBiocideOrganic active ingredientsRadiation induced apoptosisDrug overdose
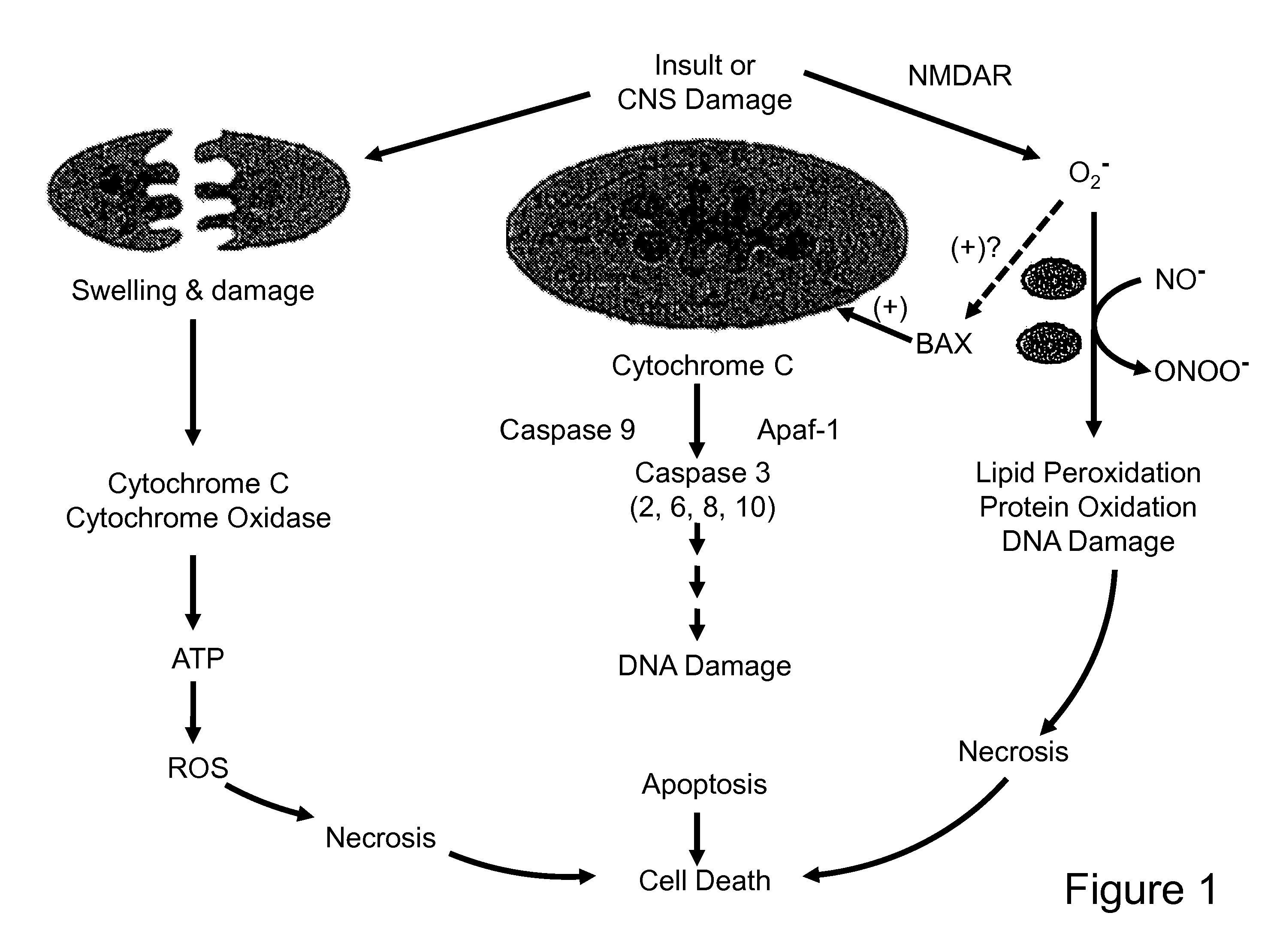
The present invention provides low molecular weight porphyrin compositions for inhibiting, preventing or delaying the binding of a ligand of a mitochondrial benzodiazepine receptor. The invention also provides pharmaceutical compositions comprising these porphyrin compositions and their use in the treatment of conditions involving the mitochondrial benzodiazepine receptor or interactions between the receptor and the mitochondrial permeability transition pore e.g., drug overdose or apoptosis including neural degeneration and radiation-induced apoptosis.
Owner:MALFROY CAMINE BERNARD +1
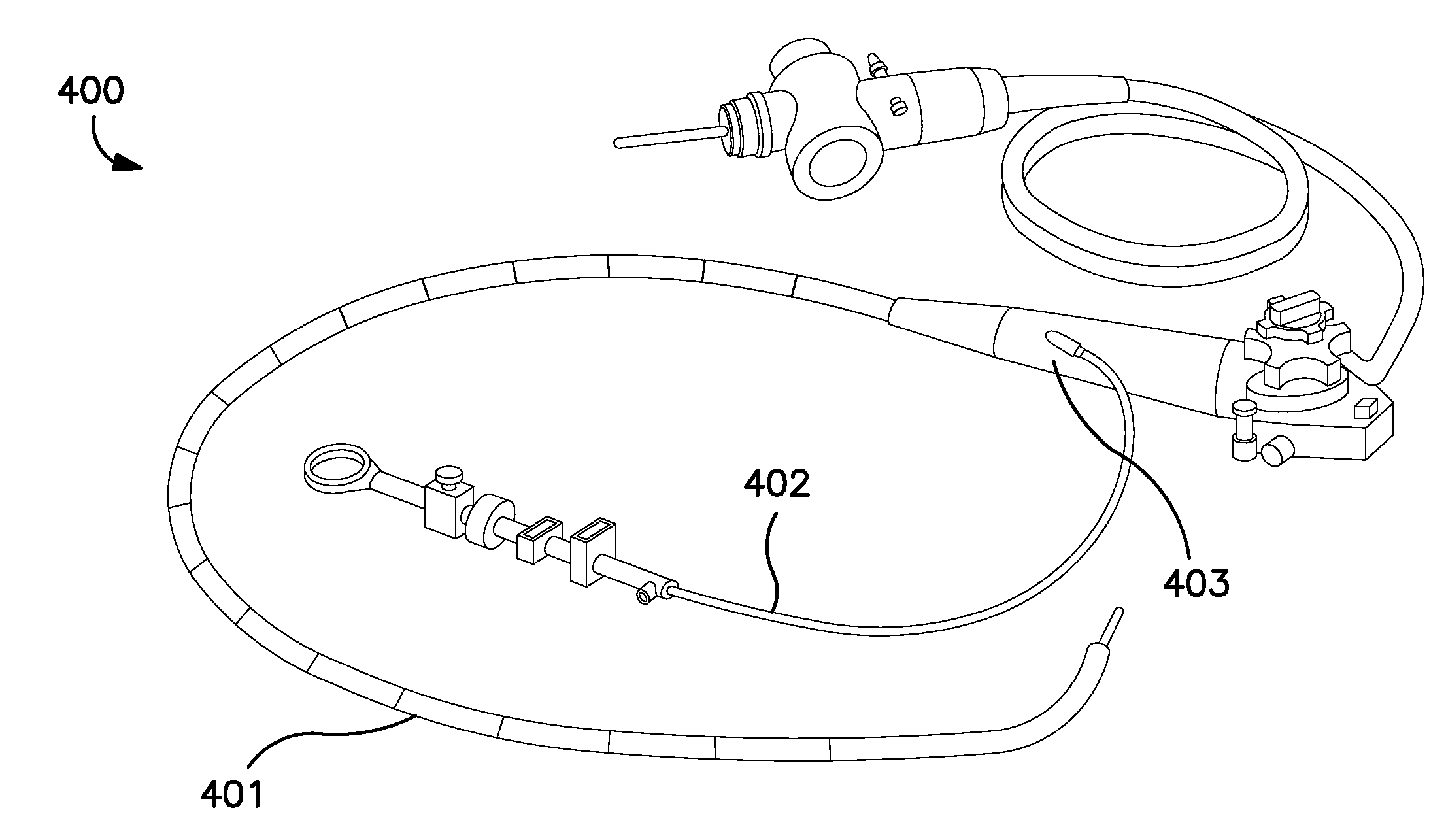
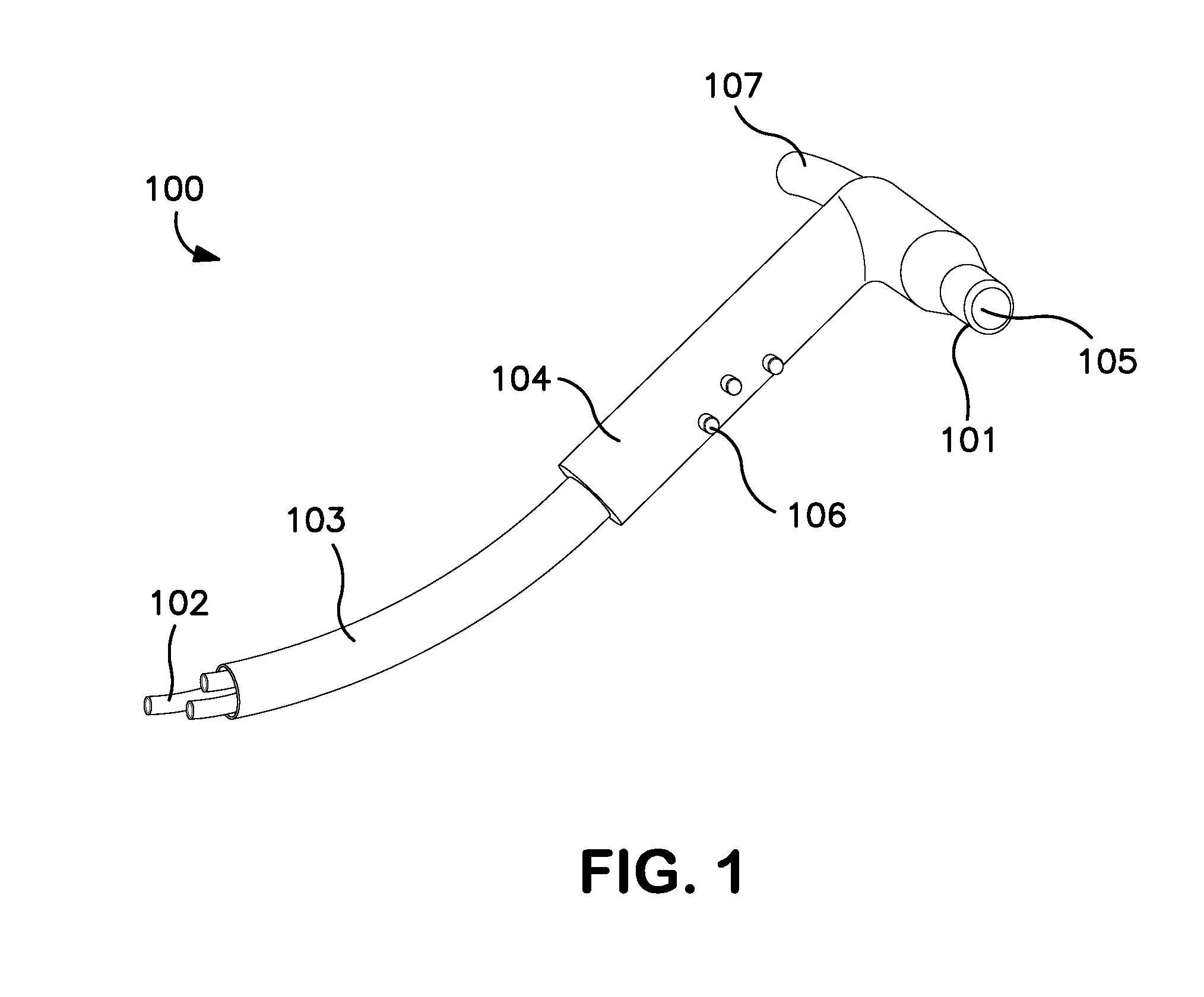
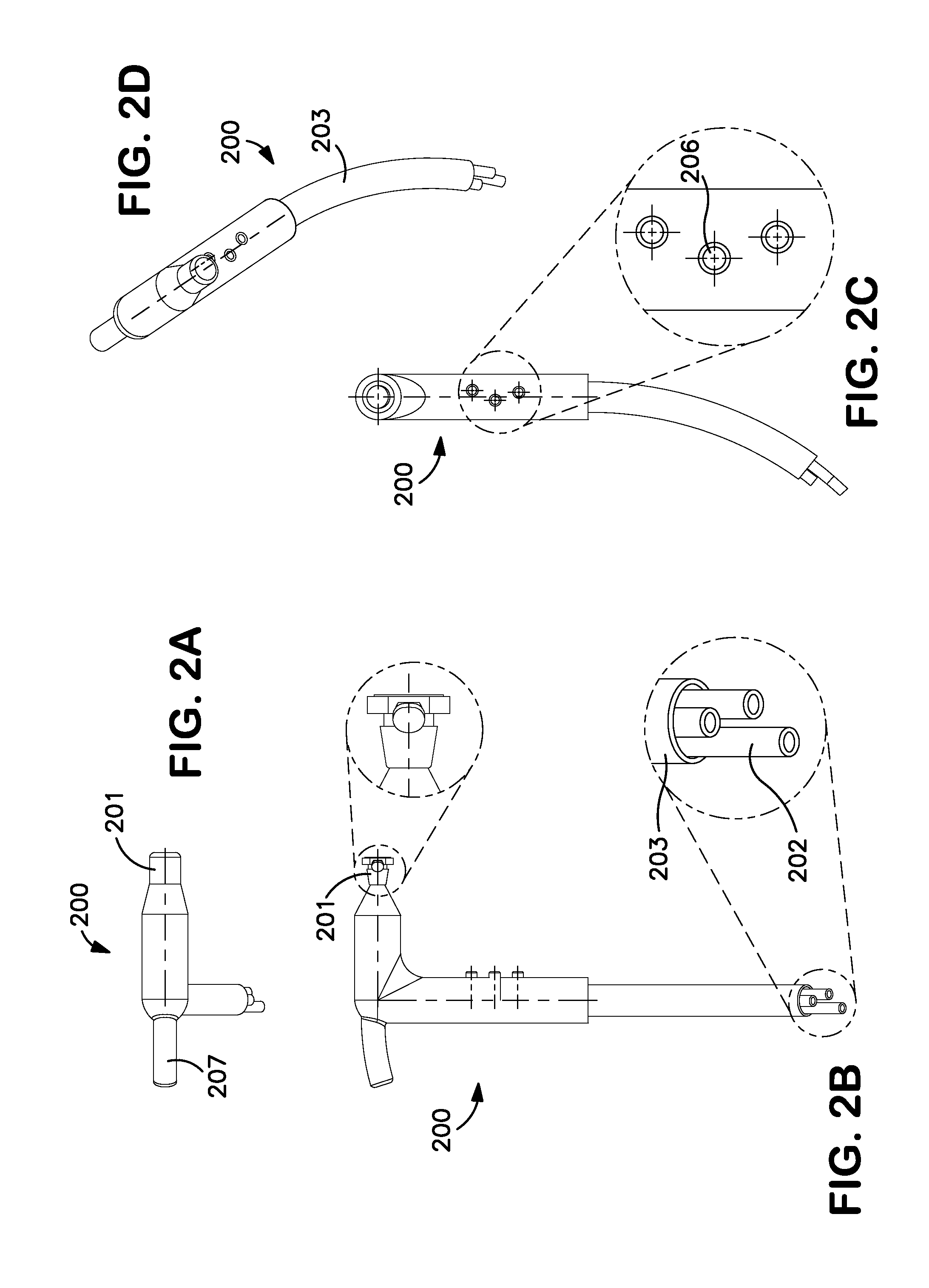
Rapid Endoscopic Gastrointestinal Irrigation System for Gastrointestinal Bleeding
InactiveUS20120130174A1Suitable for usePromote resultsSurgeryEndoscopesDrug overdoseGastrointestinal bleeding
The present invention relates to surgical instruments used in combination with endoscopes during gastrointestinal procedures for clearing a surgical site of unwanted obstructions, such as blood and / or clots. More particularly, the irrigation devices according to the invention comprise a handle comprising valves for controlling fluid flow, at least one channel for transporting fluid, and a connection port adapted to communicate directly or indirectly with an endoscope for delivering fluid to and receiving fluid from and through the endoscope. The irrigation devices and systems incorporating them can be used in any endoscopic gastrointestinal surgical procedure to clean, clear, or evacuate an area of the gastrointestinal tract, such as the stomach, in cases of accidental poisoning, drug overdose, or gastrointestinal bleeding. The irrigation device according to the present invention is capable of clearing a stomach within seconds or up to 1-2 minutes.
Owner:EDWARD VIA VIRGINIA COLLEGE OF OSTEOPATHIC MEDICINE
Enantiomerically pure opioid diarylmethylpiperazine as a cardioprotection agent
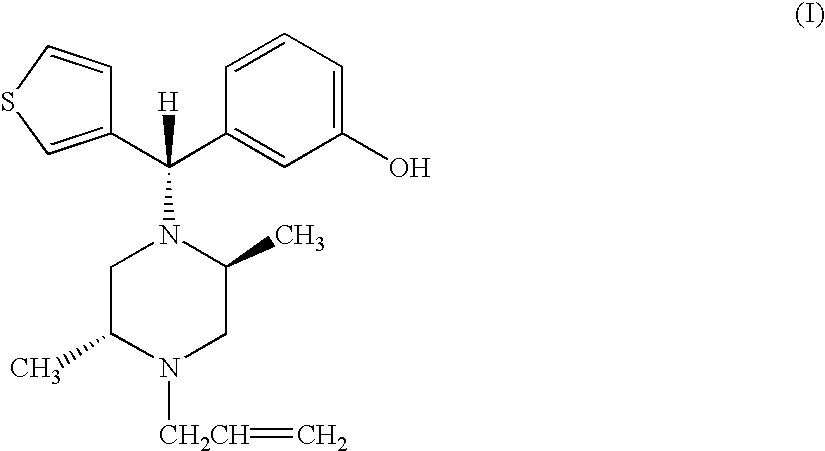
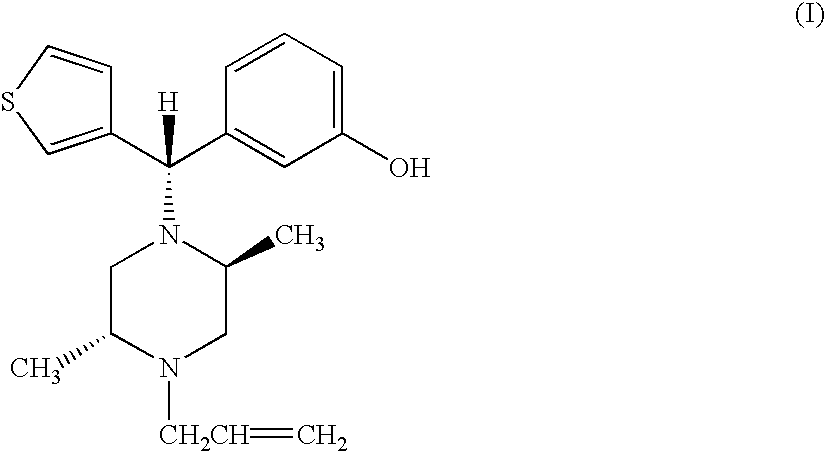
(−)3-((S)-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)(3-thienyl)methyl)phenol and pharmaceutically acceptable esters or salts thereof, in essentially enantiomerically pure form have utility as a therapeutic agent for mediating analgesia and treating cardiac disorders, alcohol addiction, drug overdose, cough, lung edema, diarrhea, respiratory, and gastro-intestinal disorders.
Owner:DMK PHARM CORP +1
Opioid overdose monitoring
An overdose of opioids can cause the user to stop breathing, resulting in death. A physiological monitoring system monitors respiration based on oxygen saturation readings from a fingertip pulse oximeter in communication with a smart mobile device and sends opioid monitoring information from the smart mobile device to an opioid overdose monitoring service. The opioid overdose monitoring service notifies a first set of contacts when the opioid monitoring information indicates a non-distress stats and notifies a second set of contact when the opioid monitoring information indicates an overdose event. The notification can be a phone call or text message to a specified person, emergency personnel, or first responders, and can include the location of the smart mobile device. The smart mobile device can also include the location of the nearest treatment center having emergency medication used in treating opioid overdose, such as naloxone.
Owner:MASIMO CORP
Enantiomerically pure opioid diarylmethylpiperazine
(−)3-((S)-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)(3-thienyl)methyl)phenol and pharmaceutically acceptable esters or salts thereof, in essentially enantiomerically pure form have utility as a therapeutic agent for mediating analgesia and treating cardiac disorders, alcohol addiction, drug overdose, cough, lung edema, diarrhea, respiratory, and gastro-intestinal disorders.
Owner:ENTA HLDG
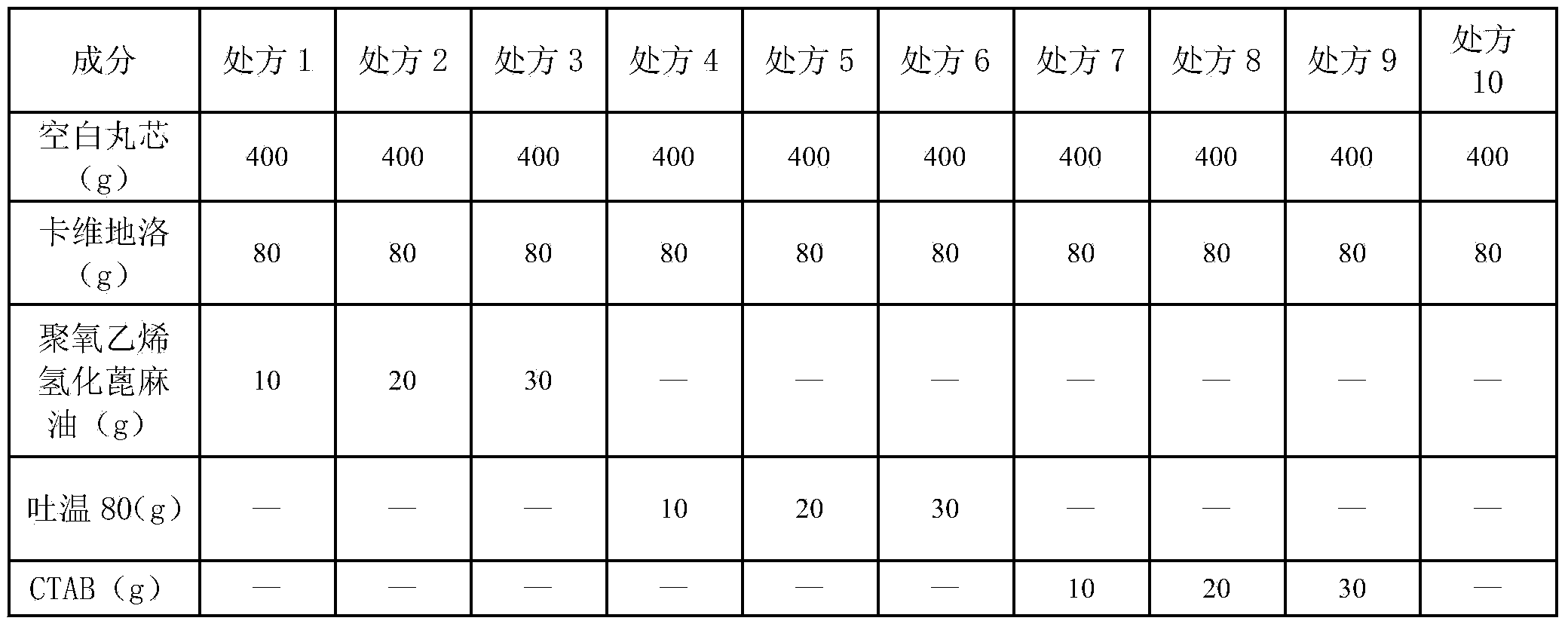
Carvedilol sustained-release capsule
The invention relates to the field of pharmaceutical preparations, in particular to a carvedilol sustained-release capsule. The carvedilol sustained-release capsule is characterized by consisting of a drug-containing pill core and a sustained-release layer, wherein the drug-containing pill core contains a blank pill core and carvedilol; the sustained-release layer contains a film-forming material and a pore-forming agent; the drug-containing pill core also contains a solubilizer; a weight ratio of the solubilizer to the active ingredients is (1:8)-(3:8); the sustained-release layer accounts for 12.6-16.1 percent of the total weight of the micro pill; the pore-forming agent consists of a methacrylic acid-ethyl acrylate (1:1) copolymer and a methacrylic acid-ethyl acrylate (1:2) copolymer; the pore-forming agent accounts for 6-11 percent of the total weight of the sustained-release layer. According to the carvedilol sustained-release preparation, only a micro pill is needed to be prepared, and a sustained-release effect can be achieved. Moreover, the medicine is slowly and fully released in main absorption parts at the pH of 5.5-6.8, the drug action time is prolonged, and the phenomenon that the safety risk of a patient is increased due to the local drug overdose caused by drug short-rate pulse release is avoided.
Owner:HEFEI HEYUAN PHARM TECH CO LTD
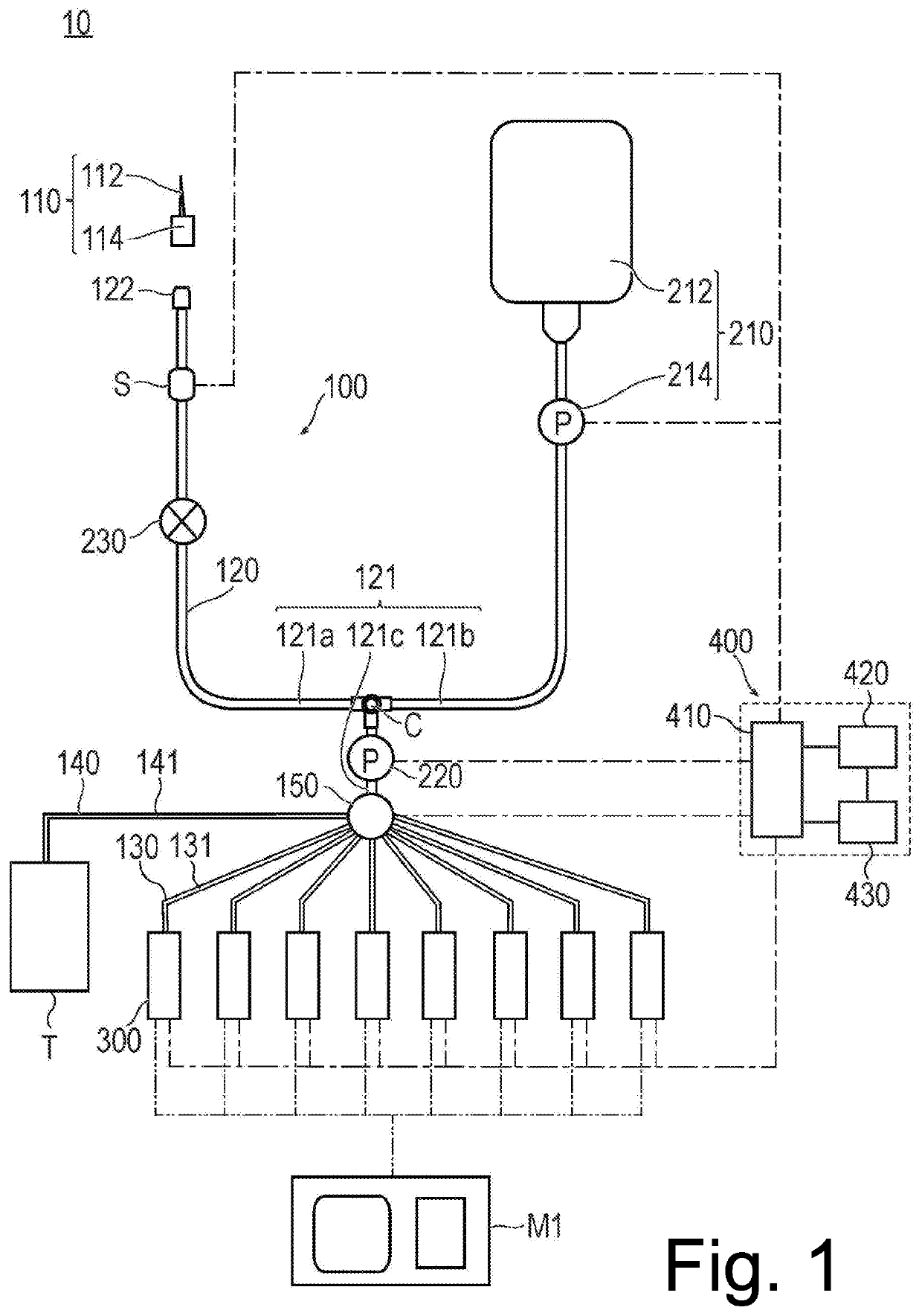
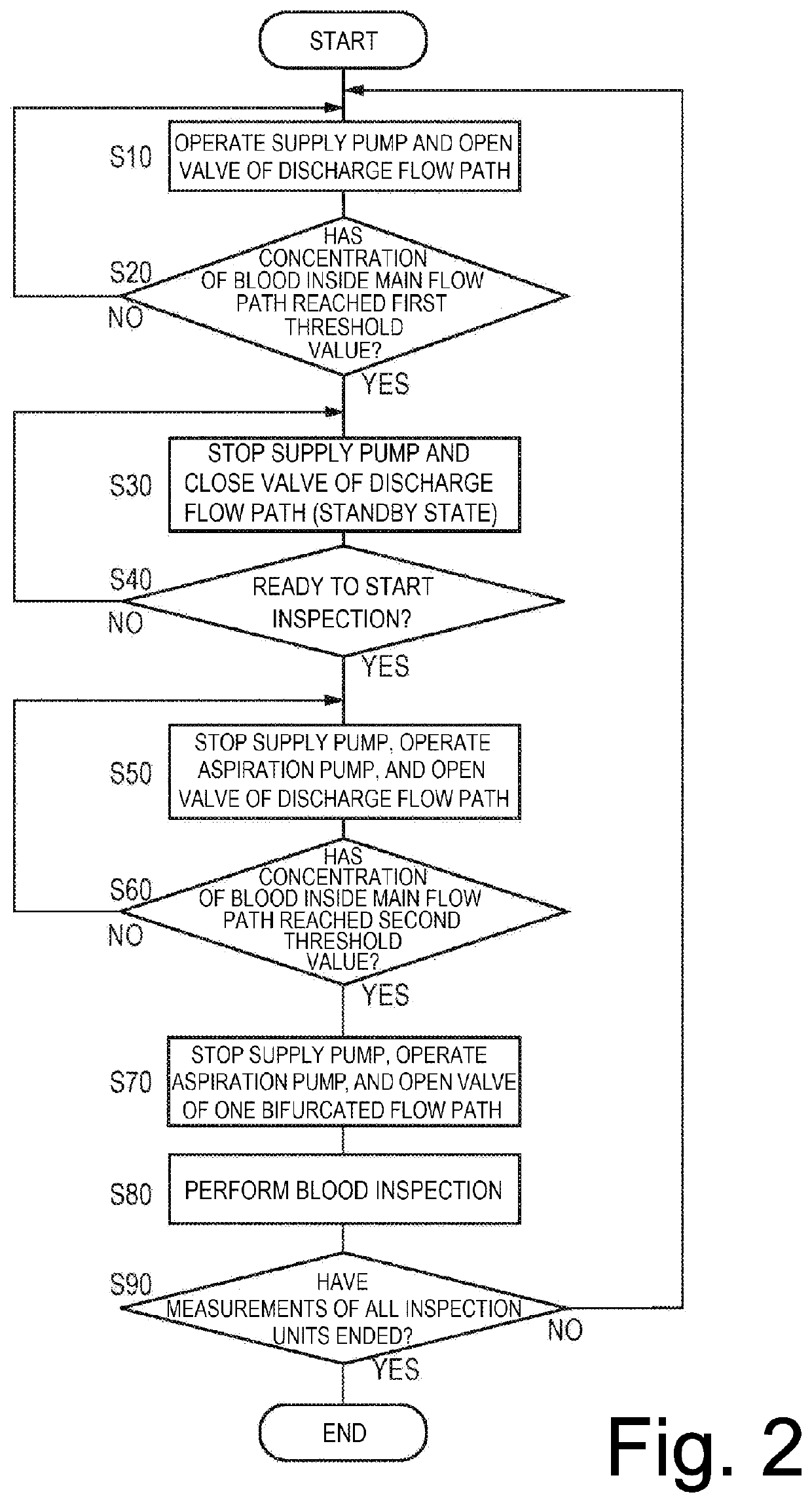
Blood inspection system and blood inspection control method
ActiveUS11318238B2Prevented from being overdosedReduce loadOther blood circulation devicesHollow article cleaningDrug overdoseThrombus
A blood inspection system automatically repeats blood inspections at a plurality of times at a desired interval and a desired timing by chronologically coupling a supply of blood to each one of a series of blood inspection units. A change in a blood condition, such as a clotting time, is monitored so that a thrombus or the like is prevented from being formed and an associated medicine is prevented from being overdosed. The blood inspection system has a catheter providing a main flow path, a supply of a flushing liquid, a plurality of inspection units, a plurality of branched flow paths, an aspiration unit, and a switching valve for selectively coupling the main flow path to a determined inspection unit which has not yet performed an inspection.
Owner:TERUMO KK
Anti-Apoptotic Benzodiazepine Receptor Ligand Inhibitors
InactiveUS20110136774A1Sufficient stability and solubility and oral bioavailabilityLow toxicityOrganic active ingredientsBiocideRadiation induced apoptosisDrug overdose
The present invention provides low molecular weight porphyrin compositions for inhibiting, preventing or delaying the binding of a ligand of a mitochondrial benzodiazepine receptor. The invention also provides pharmaceutical compositions comprising these porphyrin compositions and their use in the treatment of conditions involving the mitochondrial benzodiazepine receptor or interactions between the receptor and the mitochondrial permeability transition pore e.g., drug overdose or apoptosis including neural degeneration and radiation-induced apoptosis.
Owner:MALFROY CAMINE BERNARD +1
Enantiomerically pure opioid diarylmethylpiperzine and methods of using same
InactiveUS20030114462A1Highly efficacious for mediatingEffective strikeOrganic active ingredientsNervous disorderDiseaseHeart disease
(-)3-((S)-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)(3-thienyl)methyl)phenol and pharmaceutically acceptable esters or salts thereof, in essentially enantiomerically pure form have utility as receptor-binding species, e.g., as therapeutic agents for mediating analgesia; as co-administered agents with various other bioactive compositions, including anesthetics, barbiturates, analgesics, etc., for reducing, treating, reversing or preventing drug-mediated respiratory depression that may be directly or indirectly caused by use of such various bioactive compositions; as a conjugate in agonist / antagonist pairs for verifying / assaying receptor and neurotransmitter function; and as a therapeutic agent having utility in combating drug addiction, cardiac disorders, alcohol addiction, drug overdose, cough, lung edema, diarrhea, respiratory, and gastro-intestinal disorders.
Owner:ENTA HLDG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com