Carvedilol sustained-release capsule
A slow-release layer and slow-release pellet technology, which can be applied to non-active ingredients in medical preparations, cardiovascular system diseases, organic active ingredients, etc. bigger problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of drug-containing pellet cores
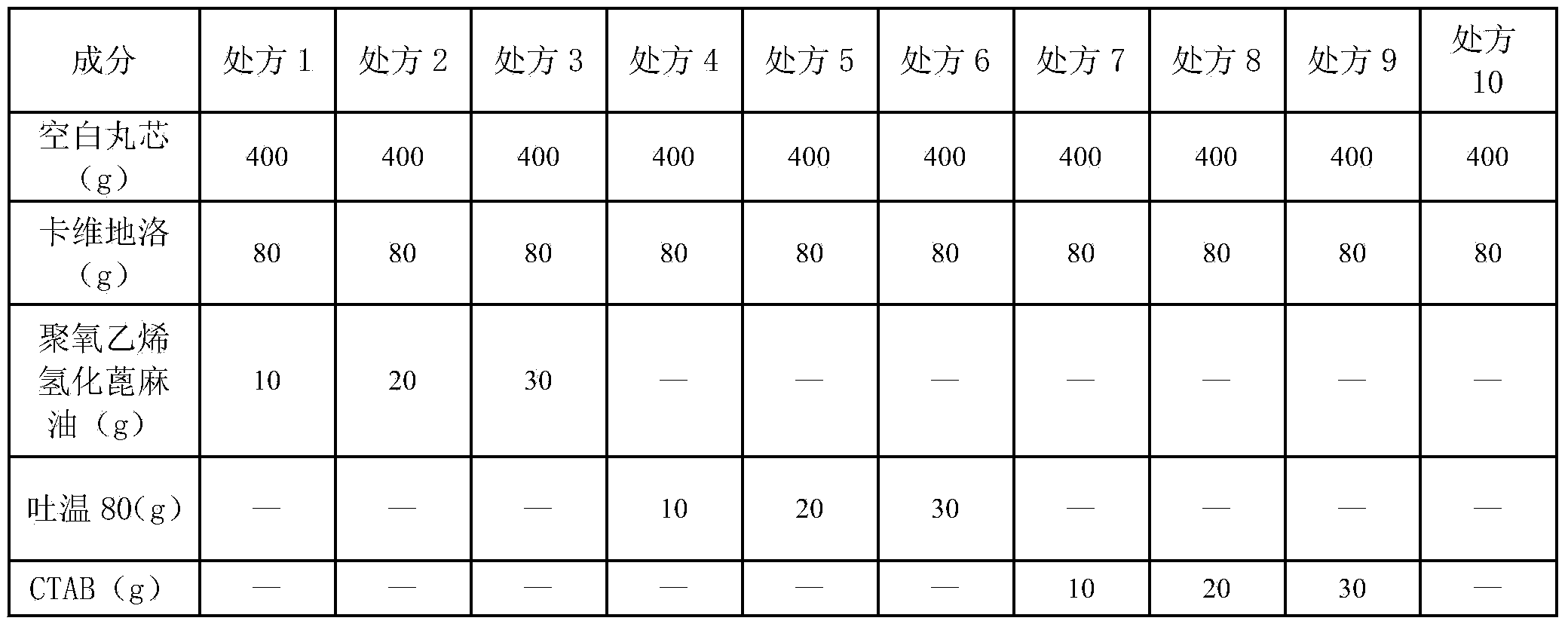
[0044]Get carvedilol 80g, polyoxyethylene hydrogenated castor oil 10g is placed in 400ml75% ethanol aqueous solution, stir to make it suspend, adopt fluidized bed (multifunctional fluidized bed test machine WBF-1, Chongqing Yingge coat coating manufacture) Granule Technology Co., Ltd.) on the blank pellet core 400g (0.3 ~ 0.5mm), the spray speed is 5rpm / min, the air inlet temperature is 40℃, and the air inlet volume is 80m 3 / min, the material temperature is 28°C, and the atomization pressure is 0.2Mpa. Keep the solution in a stirring state during the spraying process, and continue to maintain the fluidized state for 15 minutes after the drug is applied.
Embodiment 2
[0046] Preparation of pill-containing cores
[0047] Get carvedilol 80g, polyoxyethylene hydrogenated castor oil 10g is placed in 400ml75% ethanol aqueous solution, stir to make it suspend, adopt fluidized bed (multifunctional fluidized bed test machine WBF-1, Chongqing Yingge coat coating manufacture) Granule Technology Co., Ltd.) on the blank pellet core 400g (0.3 ~ 0.5mm), the spray speed is 5rpm / min, the air inlet temperature is 40℃, and the air inlet volume is 80m 3 / min, the material temperature is 28°C, and the atomization pressure is 0.2Mpa. Keep the solution in a stirring state during the spraying process, and continue to maintain the fluidized state for 15 minutes after the drug is applied.
Embodiment 3
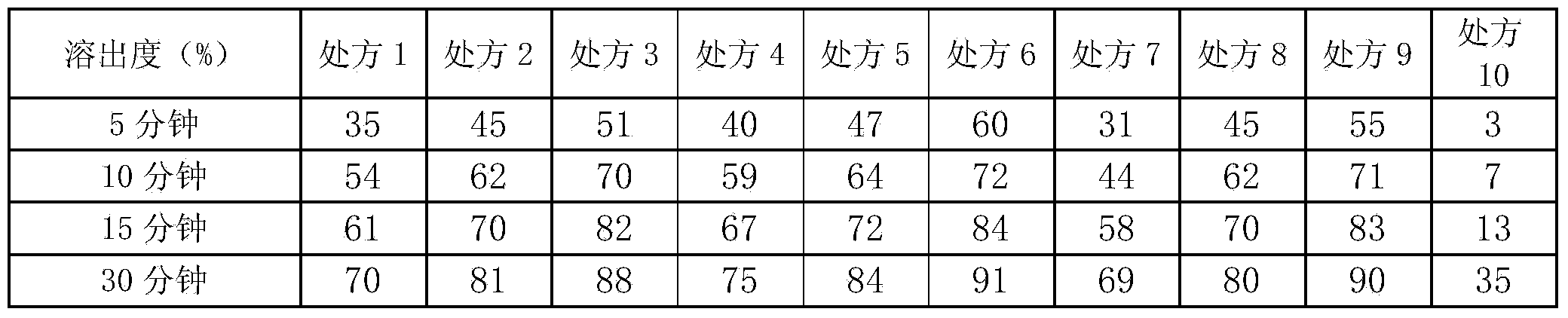
[0049] Get Eudragit NE30D aqueous dispersion 100g, wherein solid content is 30%, solvent is water, adds plasticizer triethyl citrate 7.5g wherein, antitack agent glyceryl monostearate 7.5g, porogen Eudragit L100-553g and Eudragit S1006g were uniformly dispersed at high speed, and the drug-containing pellets prepared in Example 1 were used as pellet cores, and the fluidized bed equipment was used for coating. The air inlet temperature is 25°C, and the air inlet volume is 80m 3 / min, the material temperature is 20°C, the atomization pressure is 0.25Mpa, the pumping speed of the peristaltic pump is 8rpm / min, and the coating weight gain is 10.8%. After the coating is completed, put it into an oven for aging at 40°C and sprinkle 0.5% micropowder silica gel into it. During the aging process, the pellets stick together, take them out after 2 hours, measure the content, and put the pellets into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com