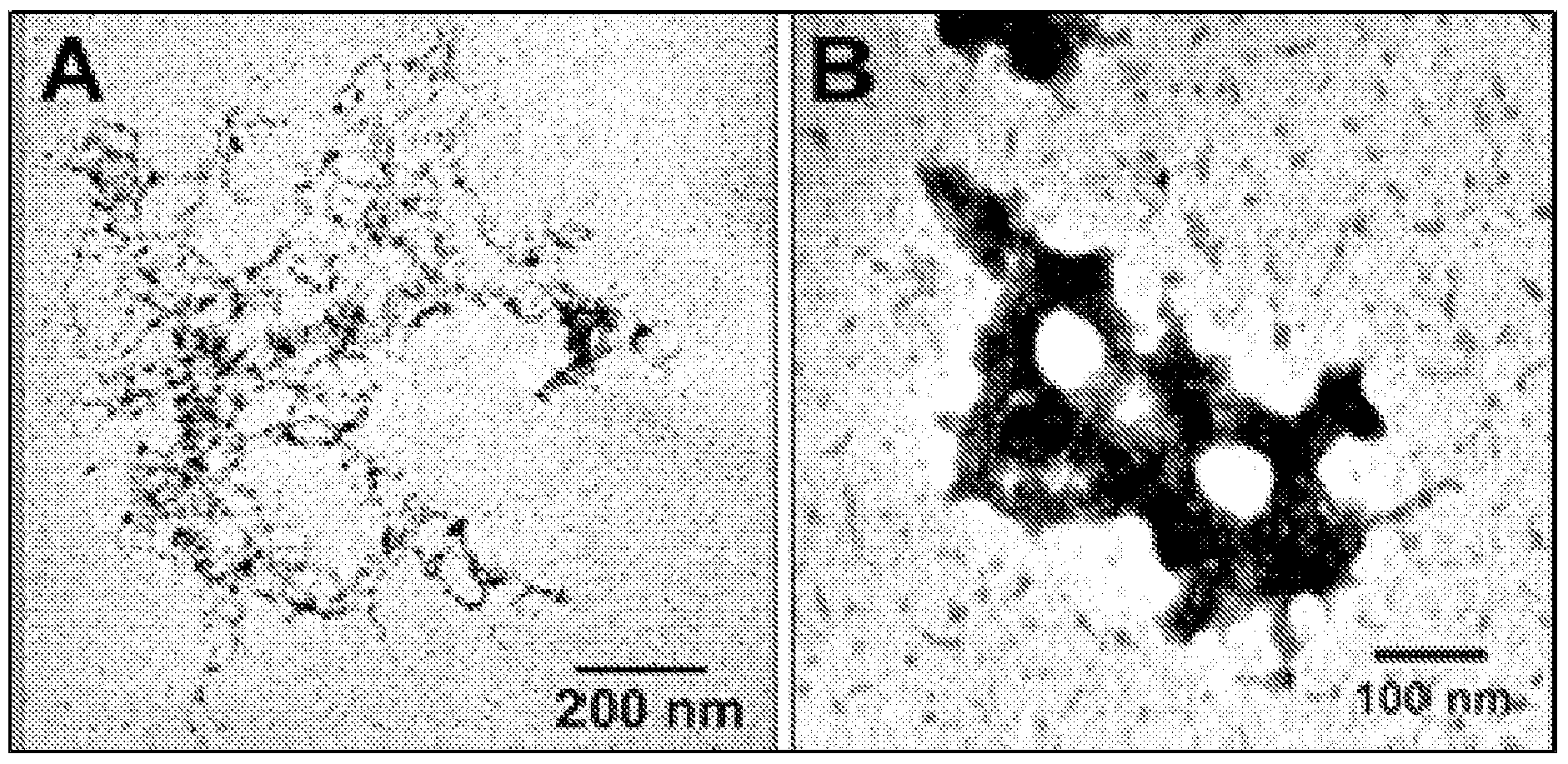
Nts-polyplex nanoparticles system for gene therapy of cancer
A cancer and compound technology, applied in the manufacture of gene therapy compositions, gene therapy, other methods of inserting foreign genetic materials, etc., can solve problems such as system inappropriateness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1.NTS-vector: Synthesis of NTS-(PF)-SPDP-PLL
[0096]For the synthesis of NTS-vectors, we used the following peptides: NTS (sequence ELYENKPRRPYIL), >90% pure, 1,672 Da molecular weight (MM) (Sigma, St. Louis MO, USA); and PF (sequence GLFEAIAEFIEGGWEGLIEGSKKK), 96% pure , 2,695 Da MM (Synpep Corp., Dublín, CA, USA). Both peptides bound simultaneously to PLL (Sigma, St. Louis MO, USA) with MM 25,600-47,900 (mean 36,750 Da). This has 251 potentially reactive amino groups. As a biofunctional crosslinker, we use N-succinimidyl 6-3[3-(2-pyridyldithio)propionamide]hexanoate (LC-SPDP; MM452.52; Cat.21651; Pierce Chemical Co, Rockford, IL, USA).
[0097] NTS-carrier synthesis is a process involving five sequential steps at room temperature: 1) formation of PLL-SPDP conjugate; 2) formation of PLL-SPDP-SH conjugate; 3) formation of NTS-SPDP conjugate; 4) Formation of PFSPDP; and 5) Formation of NTS-carrier [NTS-(PF)-SPDP-PLL] from conjugates previously obtained usi...
Embodiment 2
[0098] Example 2. Formation of PLL-SPDP-SH conjugates
[0099] Dissolve 25 mg of PLL in 2 mL of phosphate buffered saline (PBS: 17.42 mM, deNa 2 HPO 4 , 2.58mM KH 2 HPO 4 , 150mM NaCl, 1mM EDTA, pH7.2). This was mixed with 7.5 mg of LC-SPDP previously dissolved in 30 μL of dimethyl sulfoxide (DMSO). The mixture of PLL and LC-SPDP was incubated for 30 min under continuous agitation. After this time, the resulting conjugate (PLL-SPDP) was purified on an Econo-Pac10DG column (Bio-Rad Laboratories, Hercules, CA, USA) equilibrated with PBS. We collected fractions of 1 mL. 3 μl aliquots were taken from each fraction and read in a NanoDrop spectrophotometer (NanoDrop Technologies ND_1000) to determine absorbance at 215 and 280 nm.
[0100] The Econo-Pac10DG column allows molecules <6,000 Da to be eluted; thus, the PLL-SPDP conjugate (52,043 Da) is obtained in the first peak (3-7 mL) and released in the second peak (8-13 mL). Free SPDP (425.5 Da) and N-hydroxysuccinimide (114 ...
Embodiment 3
[0102] Example 3. Formation of NT-SPDP conjugates
[0103] 10 mg of NTS was dissolved in 2 mL of PBS and mixed with 5 mg of LC-SPDP previously dissolved in 30 μL of DMSO. The reaction mixture was incubated for 30 minutes with constant stirring. After incubation, the NTS-SPDP conjugate was purified on a Sephadex G-10 column (Pharmacia Fine Chemicals AB, Uppsala, Sweden) equilibrated with PBS. 0.1 mL fractions were collected; 3 μl aliquots of each fraction were read on the NanoDrop to determine their absorbance at 215 and 280 nm. Sephadex G-10 has the ability to avoid <700 Da; thus, the NTS-SPDP conjugate (2,419 Da) elutes in the first peak volume (3.5-8.4 mL). Fractions corresponding to the first peak were collected and concentrated to 1 mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com