Antifreeze polypeptide prepared by utilizing alkaline protease to carry out enzymolysis on collagens from fish skins
A technology of antifreeze peptides and amino acids, applied in peptide preparation methods, peptides, organic chemistry, etc., can solve problems such as loss of quality, damage to cell and tissue structures, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
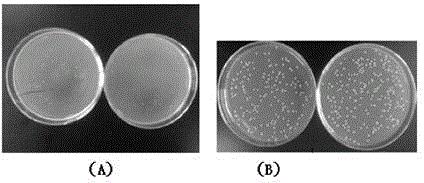
[0028] The antifreeze activity detection system for preparing antifreeze polypeptides of the present invention uses low-temperature freeze-thawed bacteria to detect the growth protection effect of adding antifreeze polypeptides on bacteria after low-temperature freeze-thaw. Inoculate the activated Lactobacillus bulgaricus into the liquid medium at 37°C, and cultivate overnight on a shaker at 130r / min as the seed liquid, inoculate the seed liquid into a new liquid medium at a ratio of 1:100, at 37°C, 130r / min Shaker culture to OD 600 =1.0 or so. Take a number of 1.5mL sterilized centrifuge tubes, add 900μL of antifreeze peptide concentration of 250μg / mL tested sample that has been filtered and sterilized, and dilute the bacterial solution by 10 4times, pipette 100 μL and add it to the sample to be tested, mix evenly, spread, incubate upside down in an incubator at 37°C for 18 hours, and count the number of colonies. Put the remaining sample-bacteria solution mixture at -20°C ...
Embodiment 1
[0034] Weigh 1.65 g of fish skin collagen and dissolve it in 6 ml of Mili-Q water, then adjust its pH to 9.0 with 2 mol / L NaOH. First heat the solution to 45°C in a water bath, then add a corresponding amount of alkaline protease according to the enzyme-substrate ratio of 1:20, and the enzymatic hydrolysis time is 30 minutes. Then inactivate the enzyme in a boiling water bath for 10 minutes, then centrifuge at 14000 rpm for 10 minutes after cooling, and collect the supernatant for later use.
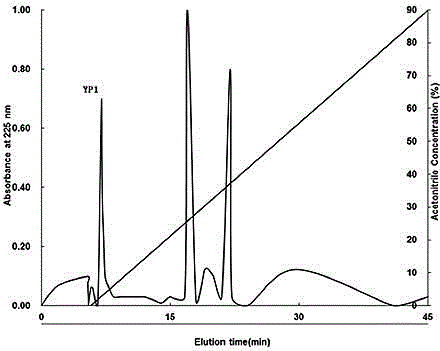
[0035] The supernatant was separated by Sephadex G-50 gel chromatography (length 100cm, diameter 2.6cm), the eluent was deionized water, the flow rate was 2mL / min, and the elution peak was measured at 225nm. Elution peak of antifreeze activity.
[0036] The elution peak with the best antifreeze activity separated by Sephadex G-50 gel chromatography is separated in the next step, separated by Sulfopropyl-Sepadex C-25 cation exchange chromatography (length 55cm, diameter 2.0cm), and elute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com