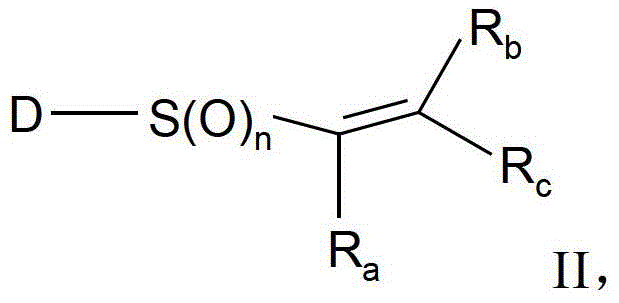
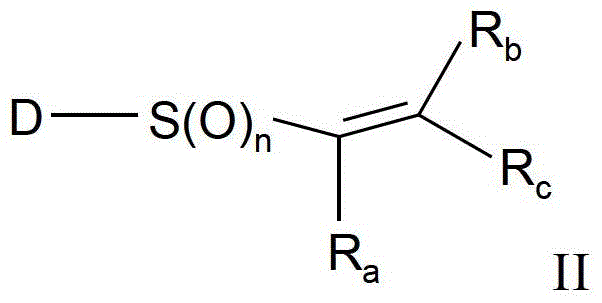
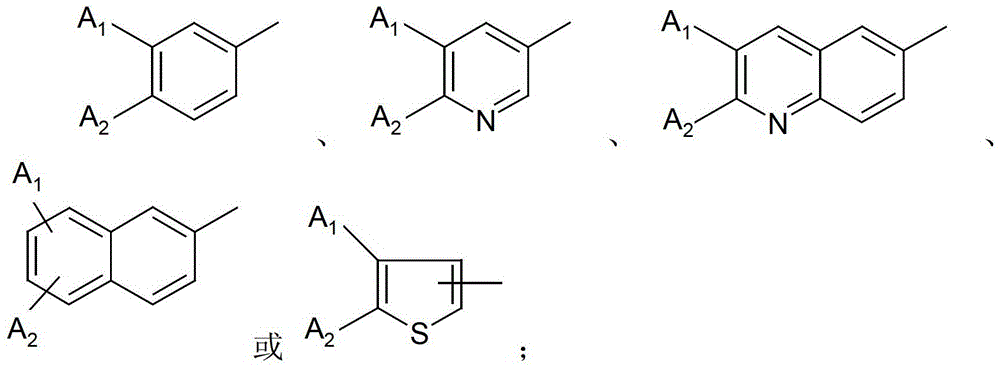
Substituted aromatic sulfur compounds and methods of use
A compound, drug technology, applied in the field of substituted aromatic sulfur compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] 2-[4-((E)-2-Cyano-vinylsulfonyl)-phenyl]-2-methyl-propionic acid
[0150]
[0151] To a solution of 2-(4-bromophenyl)-2-methylpropanoic acid (2.43g, 10mmol) in anhydrous THF (150ml) was added slowly in toluene under nitrogen at -80°C (ether / dry ice). Phenyllithium (1.8M, 11mmol) in . After 5 min, n-BuLi (2.5M in hexane, 11 mmol) was added to the mixture. A cloudy suspension slowly formed. 20 minutes after the addition of BuLi, the SO 2 Flow was bubbled through the mixture for 15 min. The reaction mixture was then allowed to warm to room temperature and the solvent was removed in vacuo. The sulfinate residue was dissolved in water (15ml), acetic acid (8ml) and MeOH (20ml), then 2-chloroacrylonitrile (18mmol) was added. The resulting mixture was stirred overnight at room temperature. The organic solvent was removed in vacuo and the residue was diluted with 20ml of water. Use saturated K 2 HPO 4 aqueous solution was adjusted to pH 5-6, then extracted with dichl...
Embodiment 2
[0153] 2-[3-((E)-2-Cyano-vinylsulfonyl)-phenyl]-2-methyl-propionic acid
[0154]
[0155] in N 2 To a solution of 2-(3-bromophenyl)-2-methylpropanoic acid (7.3g, 30mmol) in anhydrous THF (300ml) was added slowly in toluene at -80°C (ether / dry ice). Phenyllithium (1.8M, 33mmol). After 5 min, n-BuLi (2.5M in hexane, 33 mmol) was added to the mixture. A cloudy suspension slowly formed. 20 minutes after the addition of BuLi, the SO 2 Flow was bubbled through the mixture for 15 min. The reaction mixture was then allowed to warm to room temperature and the solvent was removed in vacuo. The sulfinate residue was dissolved in water (50ml), acetic acid (25ml) and MeOH (50ml), then 2-chloroacrylonitrile (60mmol) was added. The resulting mixture was stirred overnight at room temperature. The organic solvent was removed in vacuo, and the residue was diluted with 50 ml of water. Use saturated K 2 HPO 4 Aqueous solution was adjusted to pH~5-6, then extracted with dichloromethan...
Embodiment 3
[0157] (E)-3-[4-(1,1-Dimethyl-2-morpholin-4-yl-2-oxo-ethyl)-benzenesulfonyl]-acrylonitrile
[0158]
[0159] 2-[4-((E)-2-cyano-vinylsulfonyl)-phenyl]-2-methyl-propionic acid (50.0mg, 0.179mmol), morpholine (0.0156mL, 0.179mmol), N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (34.3 mg, 0.179 mmol) and 1-hydroxybenzotriazole (12.1 mg, 0.0895 mmol) were dissolved in THF (5.0 ml), and the reaction mixture was allowed to stir overnight at room temperature. The reaction mixture was poured on saturated sodium bicarbonate and the organic phase was extracted with dichloromethane / ethyl acetate. The combined extracts were then dried over sodium sulfate, filtered and concentrated. The crude reaction mixture was purified by Gilson reverse phase chromatography. The combined fractions were lyophilized to give (E)-3-[4-(1,1-dimethyl-2-morpholin-4-yl-2-oxo-ethyl)-benzenesulfonyl]- Acrylonitrile (11 mg, 17%). MS:349(M+H); 1H-NMR(DMSO-d6400MHz)δ8.24(d,1H,J=15.7Hz),7.91(d,2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com