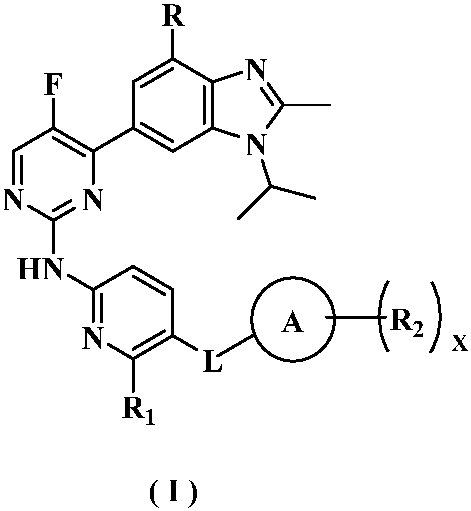
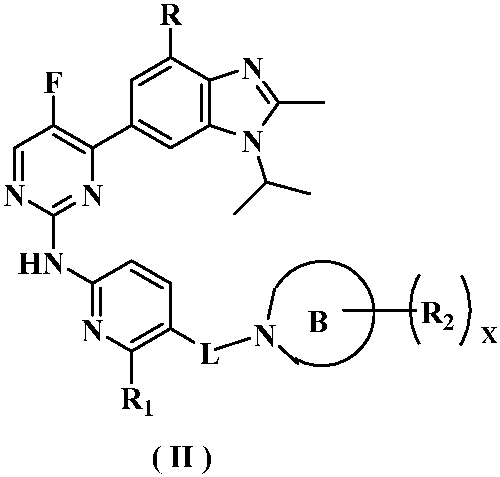
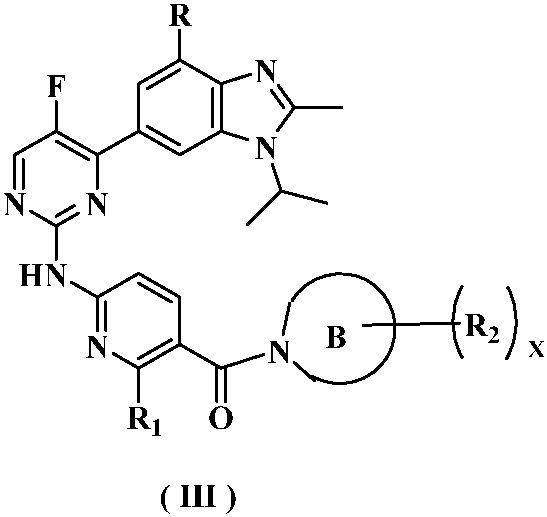
Kinase inhibitor of benzimidazole compound and its preparation method and application
A compound, a technology of a general formula compound, applied in the field of drug development, can solve problems such as good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] 1,4-diazoheptan-1-yl)(6-((5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole- Preparation of 6-yl)pyrimidin-2-yl)amino)-2-methylpyridin-3-yl)methanone
[0148]
[0149] Step 1: Preparation of tert-butyl 4-(6-chloro-2-methylnicotinyl)-1,4-diazoheptane-1-carboxylate
[0150]
[0151] 6-Chloro-2-methylnicotine acid (0.7g, 4.1mmol), tert-butyl 1,4-diazoheptane-1-carboxylate (1.0g, 4.9mmol), TEA (1.2g , 12.2mmol) dissolved in CH 2 Cl 2 (15 mL), HATU (1.87 g, 4.9 mmol) was added, and the reaction was stirred at room temperature for two hours. CH 2 Cl 2 (30mL) diluted with NaHCO 3 solution (30mL), washed with saturated brine (30mL), dried over anhydrous sodium sulfate, concentrated and column chromatographed [eluent: CH 2 Cl 2 ~CH 2 Cl 2 / MeOH (10:1)] to get the product tert-butyl 4-(6-chloro-2-methylnicotyryl)-1,4-diazoheptane-1-carboxylate (1.4g, yield 96%).
[0152] MS m / z(ESI): 354.1[M+H] + .
[0153] The second step: the preparation of 5-fl...
Embodiment 2
[0168] 1-(6-((5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidin-2-yl)amino) Preparation of -2-methylpyridin-3-yl)-4-(methylamino)piperidin-2-one
[0169]
[0170] The first step: preparation of (1-(6-chloro-2-methylpyridin-3-yl)-2-oxopiperidin-4-yl)carbamate tert-butyl ester
[0171]
[0172] 3-Bromo-6-chloro-2-methylpyridine (200mg, 0.969mmol), tert-butyl (2-oxopiperidin-4-yl)carbamate (249mg, 1.162mmol), Pd 2 (dba) 3 (89mg, 0.0972mmol), Xantphos (112mg, 0.194mmol), cesium carbonate (947mg, 2.907mmol) were stirred overnight in dioxane (10mL) at 100°C under nitrogen protection, cooled, and concentrated to obtain the product by column chromatography ( 31 mg, yield 9.4%).
[0173] MS m / z (ESI): 340.1, 342.1 [M+H] + .
[0174] The second step: (1-(6-((5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidine-2 Preparation of -yl)amino)-2-methylpyridin-3-yl)-2-carbonylpiperidin-4-yl)carbamate tert-butyl ester
[0175] ...
Embodiment 3
[0188] (4-(cyclopropylamino)piperidin-1-yl)(6-((5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d] Preparation of imidazol-6-yl)pyrimidin-2-yl)amino)-2-methylpyridin-3-yl)methanone
[0189]
[0190] Step 1: Preparation of 1-(6-chloro-2-methylnicotinoyl)piperidin-4-one
[0191]
[0192] 6-Chloro-2-methylnicotinic acid (1.0g, 5.8mmol), piperidin-4-one (866mg, 8.7mmol), HATU (2.2g, 17.5mmol), DIEA (2mL) were sequentially added to dichloromethane (50mL). The reaction was stirred at room temperature for 4 hours, LCMS showed that the reaction was complete, the reaction solution was separated by adding dichloromethane (50mL) and water (50mL), the organic phase was washed with saturated sodium bicarbonate (3x20mL), the organic phase was separated, and washed with anhydrous Dry over sodium sulfate, filter and concentrate. The remaining crude product was purified by flash silica gel column (CH 2 Cl 2 :MeOH=20:1) to obtain the product 1-(6-chloro-2-methylnicotinoyl)piperidi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Membrane resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com