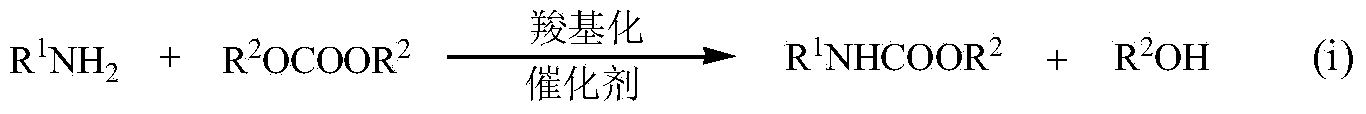Synthesis of N-methylmethyl carbamate
A technology of methyl methyl carbamate and dimethyl carbonate, which is applied in the field of synthesizing methyl N-methyl carbamate, can solve problems such as the study of un-MMC systems, and achieve economical efficiency, easy operation and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: catalyst is NaOH
[0027] 1,3-Dimethylurea (14.91g, 169.2mmol), NaOH (0.81g, 20.30mmol) and 15.23g of dimethyl carbonate were added into a 50mL stainless steel reaction kettle. At 0.1MP, 150 ° C under closed stirring reaction for 6h. The result is:
[0028] DMU conversion rate%
Embodiment 2
[0029] Embodiment 2: Catalyst is DBTO
[0030] Will Bu 2 SnCl 2 (9.48g, 31.2mmol) was dissolved in 30mL of diethyl ether, stirred, set aside, and set aside. Add 30mL of 10% NaOH solution into a 100mL three-necked flask equipped with a thermometer, a stirring distillation device, and a dropping funnel, heat it to 50°C, and slowly add the above-mentioned clear diethyl ether solution dropwise under stirring, and at the same time recover the distilled water with a distillation device. Ether was released, and after dripping, the reflux device was changed to boil the solution for 0.5h. The white precipitate was filtered off. Wash with distilled water until neutral, and dry to obtain DBTO as a white granular solid.
[0031] 1,3-Dimethylurea (1.35g, 15.34mmol), DBTO (0.15g, 0.60mmol) and 15.23g of dimethyl carbonate were added into a 50mL stainless steel reaction kettle. Dimethyl carbonate acts as both a reactant and a reaction solvent. In 0.1MP, 150 ° C closed stirring reaction...
Embodiment 3
[0033] Embodiment 3: catalyst is MgO
[0034] 1,3-Dimethylurea (1.35g, 15.34mmol), MgO (0.061g, 1.53mmol) and 15.23g of dimethyl carbonate were added into a 50mL stainless steel reaction kettle. Dimethyl carbonate acts as both a reactant and a reaction solvent. At 2.0MP, 160 ° C under closed stirring reaction for 10h. The result is:
[0035] DMU conversion rate%MMC yield%
[0036] 63.140.4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com