Multivalent combined vaccine
A combined vaccine and live attenuated vaccine technology, applied in the field of multivalent combined vaccines, can solve the problems of short-term immune protection, low vaccine protection, weak immunogenicity, etc., to reduce the number of immunization shots, reduce the probability of outbreaks, and enhance Immunogenic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
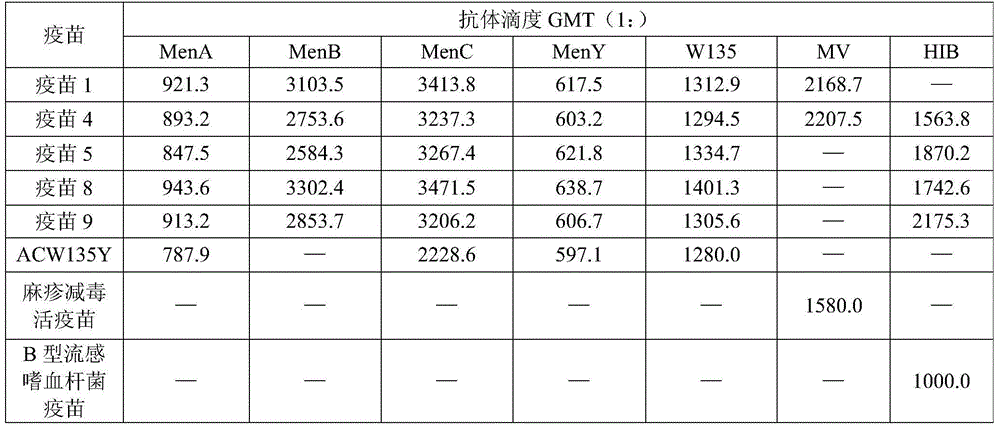
[0034] Example 1 (Vaccine 1)
[0035] Prepare A, C, Y, W135 groups of Neisseria meningitidis capsular polysaccharides, B group meningococcal live attenuated vaccine and measles live attenuated vaccine according to the aforementioned method, and each dose contains A group epidemic meningitis Neisseria capsular polysaccharide 15 μg, C group Neisseria meningitidis capsular polysaccharide 15 μg, Y group Neisseria meningitidis capsular polysaccharide 15 μg, W135 group Neisseria meningitidis capsule Membrane polysaccharide 15 μg, group B meningococcal live attenuated vaccine 20 μg, measles live attenuated vaccine 20 μg, aluminum phosphate adjuvant 1 mg, diluted by conventional methods to make 0.5ml / dose liquid dose.
Embodiment 2
[0036] Example 2 (Vaccine 2)
[0037] Prepare A, C, Y, W135 groups of Neisseria meningitidis capsular polysaccharides, B group meningococcal live attenuated vaccine and measles live attenuated vaccine according to the aforementioned method, and each dose contains A group epidemic meningitis Neisseria capsular polysaccharide 20 μg, C group Neisseria meningitidis capsular polysaccharide 20 μg, Y group Neisseria meningitidis capsular polysaccharide 20 μg, W135 group Neisseria meningitidis capsule Membrane polysaccharide 20 μg, group B meningococcal live attenuated vaccine 10 μg, measles live attenuated vaccine 10 μg, aluminum hydroxide adjuvant 0.5 mg, diluted by conventional methods to prepare a liquid dose of 0.5 ml / dose.
Embodiment 3
[0038] Example 3 (Vaccine 3)
[0039] Prepare A, C, Y, W135 groups of Neisseria meningitidis capsular polysaccharides, group B meningococcal live attenuated vaccines, measles live attenuated vaccines and Haemophilus influenzae type B capsular polysaccharides according to the aforementioned method, According to each dose, it contains 15 μg of capsular polysaccharide of Neisseria meningitidis group A, 15 μg of capsular polysaccharide of Neisseria meningitidis group C, and 15 μg of capsular polysaccharide of Neisseria meningitidis group Y, W135 Neisseria meningitidis group capsular polysaccharide 15 μg, group B meningococcal live attenuated vaccine 15 μg, measles live attenuated vaccine 10 μg, Haemophilus influenzae type B capsular polysaccharide 10 μg, aluminum phosphate adjuvant 1 mg, using Dilute it in a conventional way to make a liquid dose of 0.5ml / dose.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com