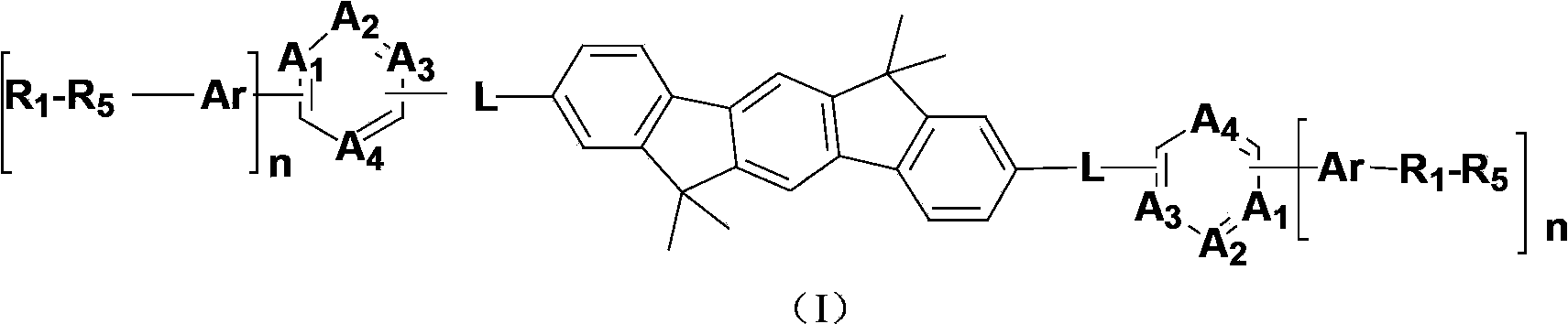
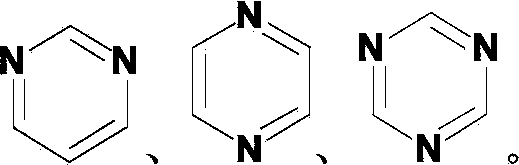
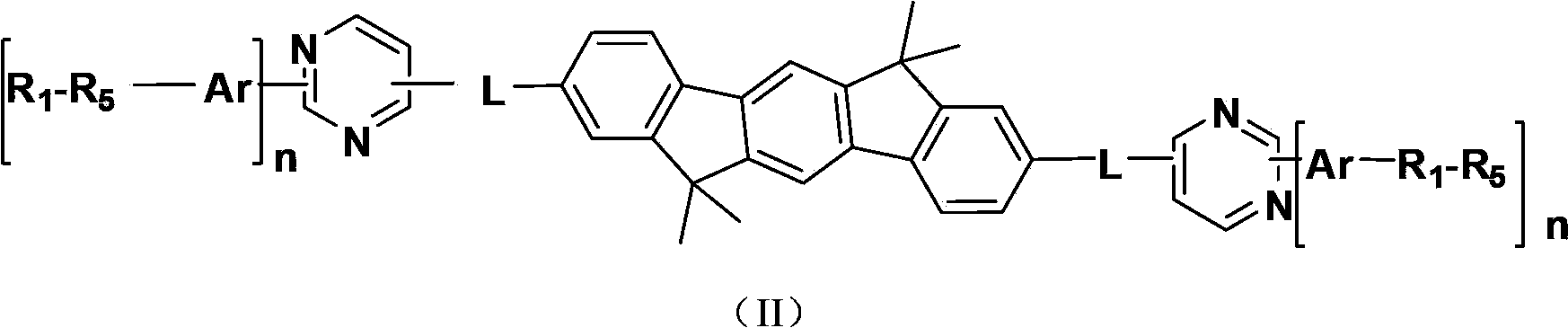
Indenofluorene derivative containing pyrimidyl or pyrazinyl or triazinyl group, and its application
A technology of derivatives and compounds, applied in the field of new organic materials, can solve the problems of increasing the complexity of the device manufacturing process, reducing the cost of OLEDs, disadvantages, etc., and achieve good thermal stability, good film-forming performance, and high electron mobility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] The synthesis of embodiment 1 compound 1
[0096] (1) The first step
[0097]
[0098] Under the protection of Ar gas, 18.2 g of 2,4,6-trichloropyrimidine (molecular weight 182, 0.10 mol), 17.92 g of indenofluorene-2,7-diboronic acid (molecular weight 398, 0.045 mol), tetrakis(triphenylphosphine) palladium 6.0g (0.0052mol), THF 600ml, toluene 400ml, potassium carbonate 60g (0.435mol) dissolved in 400ml water to form a solution into the reaction flask. After repeated ventilation under reduced pressure, start the electric stirring, monitor the reaction with TLC (thin layer chromatography), and the reaction is complete after reflux for 4 hours. After cooling, the reaction system was divided into two layers. The organic layer was separated and evaporated to dryness to obtain a solid product, which was recrystallized with toluene to obtain 23.65 g of an intermediate with a molecular weight of 604 and a yield of 87%.
[0099] (2) The second step
[0100]
[0101] Und...
Embodiment 2
[0103] The synthesis of embodiment 2 compound 2
[0104] (1) The first step
[0105]
[0106] Under the protection of Ar gas, 18.2 g of 2,4,6-trichloropyrimidine (molecular weight 182, 0.10 mol), 28.1 g of phenylboronic acid (molecular weight 122, 0.23 mol), tetrakis(triphenyl Phosphine) Palladium 12.0g (0.0104mol), 600ml of THF, 400ml of toluene, potassium carbonate 60g (0.435mol) dissolved in 400ml of water to form a solution into the reaction flask. After repeated ventilation under reduced pressure, start the electric stirring, monitor the reaction with TLC (thin layer chromatography), and the reaction is complete after reflux for 8 hours. After cooling, the reaction system was divided into two layers. The organic layer was separated and evaporated to dryness to obtain a solid product, which was recrystallized with toluene to obtain 19.9 g of an intermediate with a molecular weight of 266 and a yield of 75%.
[0107] (2) The second step
[0108]
[0109] Under the ...
Embodiment 3
[0111] The synthesis of embodiment 3 compound 3
[0112] (1) The first step
[0113]
[0114] Under the protection of Ar gas, carbazole 16.7g (molecular weight 167, 0.1mol) was dissolved in anhydrous DMF180ml, and a solution of 5.64g NaH (content 55%, 0.235mol) in 180ml DMF was added dropwise for 20 minutes and stirred for 1h. Then, 18.2g of 2,4,6-trichloropyrimidine (molecular weight 182, 0.1mol) dissolved in 180ml of DMF was added in 20 minutes, stirred for 3 hours, poured into 1000ml of water, filtered and dried in vacuum, and the product was washed with silica gel After column purification, 25.4 g of the target molecule (0.081 mol) was obtained with a molecular weight of 313 and a yield of 81%.
[0115] (2) The second step
[0116]
[0117] Under the protection of Ar gas, add 15.6g of the reaction product of the previous step (molecular weight 313, 0.05mol), 6.71g of phenylboronic acid (molecular weight 122, 0.055mol), tetrakis (triphenylphosphine) palladium 3.0 g (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com