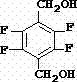
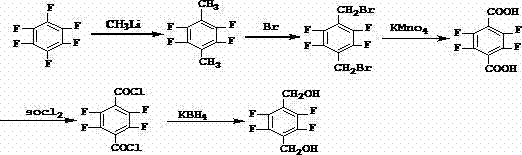
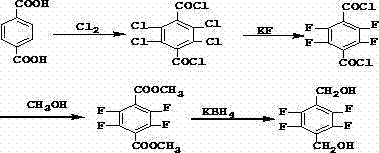
Synthetic method of 2,3,5,6-tetrafluoro-1,4-benzenedimethanol
A technology for terephthalimethanol and a synthesis method, which is applied in the field of synthesis of 2,3,5,6-tetrafluoro-1,4-terephthalenediethanol, can solve the problem of serious corrosion of production equipment, large environmental pollution, and process routes. It can reduce the consumption of raw materials, improve the environment, and reduce side reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthetic method of 2,3,5,6-tetrafluoro-1,4-p-xylylenedimethanol comprises the following steps:
[0039] a) Preparation of 2,3,5,6-tetrafluoro-1,4-terephthalonitrile
[0040] In the dry reaction flask, add 500g DMF and 160g potassium fluoride, add 162g 2,3,5,6-tetrachloro-1,4-terephthalonitrile at room temperature under stirring, raise the temperature to 110°C, and control the reaction The temperature is 110-120°C, and the reaction is kept for 6 hours. After the reaction is completed, cool to 0°C and filter with suction, and wash the filter cake with water to obtain 2,3,5,6-tetrafluoro-1,4-terephthalonitrile-like white solid. Content ≥99%, mp 195~196.5°C (literature 193~195°C).
[0041] b) Preparation of dimethyl 2,3,5,6-tetrafluoro-1,4-terephthalate
[0042] Add 145g of 95% methanol to the reaction flask, stir and add 0.2g of the existing manganese dioxide catalyst, add 140g of 2,3,5,6-tetrafluoro-1,4-terephthalonitrile, heat up to reflux, slowly Add 345g of con...
Embodiment 2
[0054] Preparation of 2,3,5,6-tetrafluoro-1,4-p-xylylenedimethanol (comparative example)
[0055] a) Preparation of 2,3,5,6-tetrafluoro-1,4-terephthalonitrile
[0056] Add 620g DMF, 135g potassium fluoride, 135g 2,3,5,6-tetrachloro-1,4-terephthalonitrile, 2.5g hexadecyltrimethylammonium bromide in the dry reaction flask, stir Raise the temperature to 125-130°C, keep it warm for 4 hours, finish the reaction, distill under reduced pressure, recover 300g of DMF, add water to dilute the material in the bottle, filter with suction to obtain a yellow solid, and refine it to obtain off-white crystals, the yield is 78.5%, the content is 98.5%, mp 195~195.5℃.
[0057] b) Preparation of 2,3,5,6-tetrafluoro-1,4-terephthalic acid
[0058] Add 80g of water into the reaction bottle, stir and add 310g of sulfuric acid and 120g of glacial acetic acid, then add 150g of 2,3,5,6-tetrafluoro-1,4-terephthalonitrile, heat up to 140-142°C and keep it warm for 10 hours. After the reaction is compl...
Embodiment 3
[0063] Embodiment 3 (comparative example)
[0064] Preparation of 2,3,5,6-tetrafluoro-p-methoxybenzyl alcohol
[0065] Add toluene and 2,3,5,6-tetrafluoro-1,4-tere-phenylenedimethanol into the reaction flask, stir at 0°C and add dimethyl sulfate and liquid caustic dropwise at the same time, raise the temperature to 30°C after dropping, and stir to react After 2 hours, the reaction was completed, separated, washed with water until neutral, and a mixture of toluene and 2,3,5,6-tetrafluoro-p-methoxybenzyl alcohol was obtained. Triethylamine was added to the mixture, stirred and dropped at 0°C. Add dichlorochrysanthemum acid chloride, stir at room temperature for 4 hours after dropping, add water, wash with water until neutral, evaporate toluene to obtain perfluthrin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com