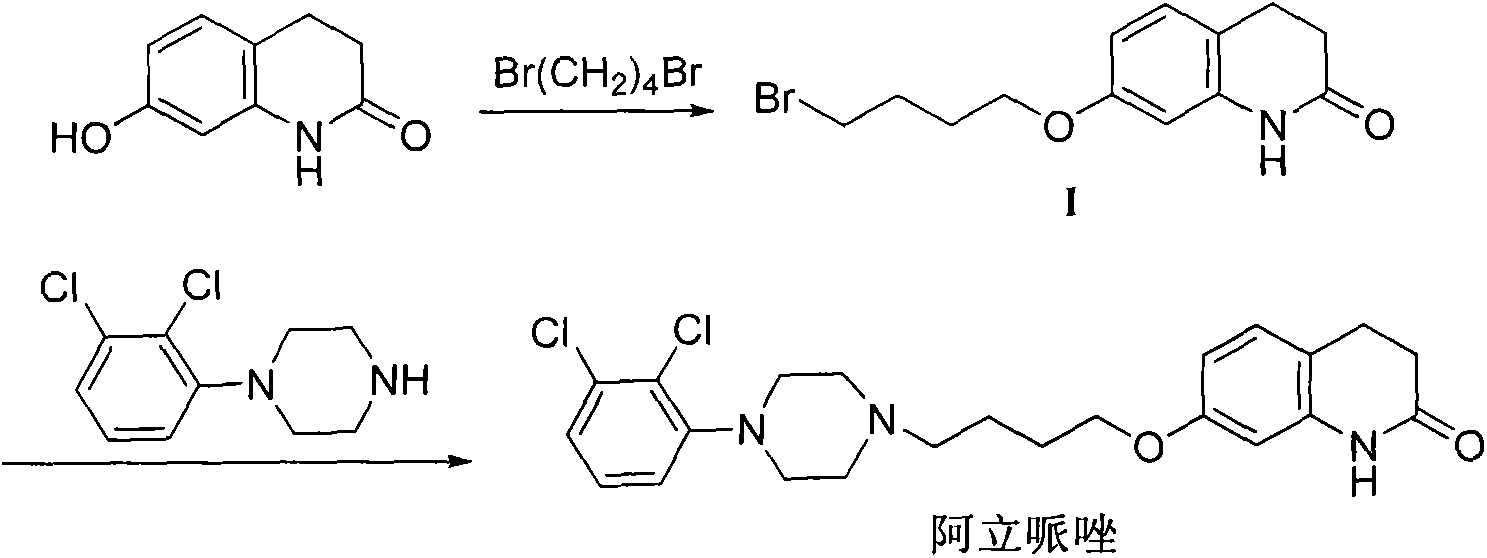
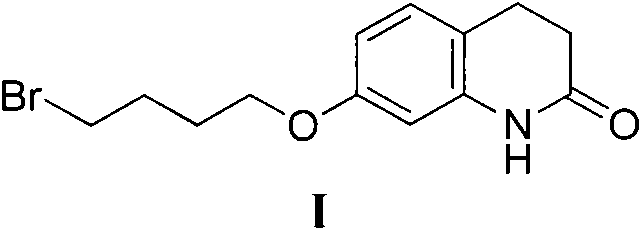
Aripiprazole intermediate synthesis method
A synthesis method and dihydrogen technology are applied in the field of medicine and chemical industry, which can solve the problems of reducing the reaction yield and achieve the effect of saving cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 7-Hydroxy-3,4-dihydro-2(1H)quinolinone (81.5g, 0.5mol), potassium carbonate (138g, 1mol), hexadecyltributylphosphine bromide (25.35g, 0.05mol ), 1,4-dibromobutane (324g, 1.5mol), and reflux reaction in 800mL methyl isobutyl ketone for 1.5 hours. Cool to room temperature, filter, and beat the filter cake with methyl tert-butyl ether for 30 minutes, combine the mother liquor and concentrate to dryness, add n-heptane, crystallize for 3 hours, and filter. Blast dried overnight to obtain 136 g of white solid with a yield of 92.3% (HPLC purity 99.2%, impurity 7-BQB, 0.58%).
Embodiment 2
[0019] 7-Hydroxy-3,4-dihydro-2(1H)quinolinone (81.5g, 0.5mol), potassium carbonate (138g, 1mol), triphenylpropylphosphine bromide (1.93g, 0.005mol), 1,4-Dibromobutane (324g, 1.5mol) was reacted in 800mL methyl isobutyl ketone at 40°C for 1 hour. Cool to room temperature, stir overnight, filter, filter the cake with methyl isobutyl ketone for 30 minutes, combine the mother liquor and concentrate to dryness, add n-heptane, crystallize for 3 hours, and filter. Blast dried overnight to obtain 135 g of white solid with a yield of 91.3% (HPLC purity 99.15%, impurity 7-BQB, 0.62%).
Embodiment 3
[0021] 7-Hydroxy-3,4-dihydro-2(1H)quinolinone (81.5g, 0.5mol), potassium carbonate (138g, 1mol), triphenylethylphosphine iodide (4.18g, 0.025mol), 1,4-Dibromobutane (324g, 1.5mol) was reacted in 800mL methyl isobutyl ketone at 70°C for 1 hour. After filtering, the filter cake was beaten with butyl acetate for 30 minutes, the combined mother liquor was concentrated to dryness, n-heptane was added, crystallized for 3 hours, and filtered. Blast dried overnight to obtain 137.8 g of white solid with a yield of 92.7% (HPLC purity 99.04%, impurity 7-BQB, 0.61%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com