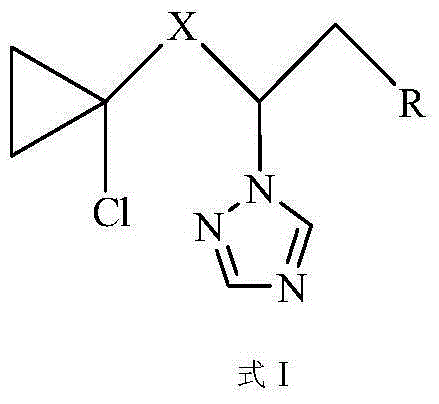
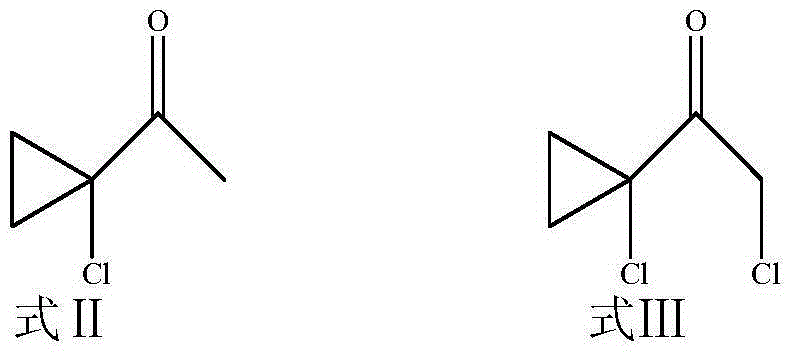A kind of aryltriazole compound containing chlorocyclopropane and its preparation method and application
A technology for substituting cyclopropanyl and aryl triazoles, which is applied in the field of aryl triazole compounds, can solve the problems of long residual effect and low selectivity, and achieve the effect of cheap raw materials, wide application value and good growth inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. Preparation and structure identification of compound CAUZJ-A-02 (X=CO; R is p-bromophenyl).
[0048] The reaction equation of the present embodiment is as follows:
[0049]
[0050] (1) Add 1-acetyl-1-chlorocyclopropane (Formula II, 10g, 0.084mol) into a 100ml round bottom flask, dissolve in 25ml of anhydrous dichloromethane, and slowly add sulfonyl chloride dropwise under ice bath conditions (8.45ml, 0.101mol), continue to react for 8h after dripping, add water to quench, extract with dichloromethane, evaporate dichloromethane after drying, and obtain 8g of light green clear liquid with pungent smell, yield 62%. That is formula III.
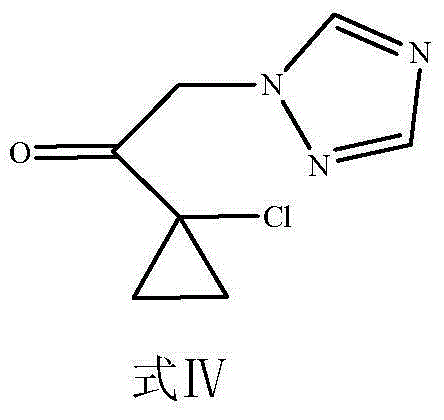
[0051] (2) Take the product 1-chloroacetyl-1-chlorocyclopropane obtained in (1), that is, formula III (5g, 0.033mol) and put it in a 250ml round bottom flask, dissolve it in 100ml of ethyl acetate, and add 1, 2,4-triazole (2.3g, 0.033mol), anhydrous potassium carbonate (5.98g, 0.043mol), tetrabutylammonium iodide (0.36g, 0...
Embodiment 2
[0056] Example 2. Preparation and structure identification of compound CAUZJ-A-13 (X=CHOH; R is p-bromophenyl).
[0057] The reaction equation prepared in this embodiment is as follows:
[0058]
[0059] Take 0.5g (1.4mmoL) of CAUZJ-A-02 into a 50ml round bottom flask, add 25ml of methanol to dissolve, add 0.1g (2.6mmoL) of sodium borohydride under ice bath conditions, and react for 3 hours. After the reaction, add water, extract with ethyl acetate, dry and recrystallize to obtain 0.35g CAUZJ-A-13, the yield is 70%, m.p.96℃
[0060] Structural Confirmation Data:
[0061] 1 H NMR (300MHz, CDCl 3 )δ0.426-0.507(m,1H,CH 2 ),0.708-0.789(m,1H,CH 2 ),1.005-1.085(m,1H,CH 2 ),1.102-1.255(m,1H,CH 2 ),3.271-3.298(d,2H,CH 2 Ar),3.955-3.970(d,1H,CHOH),4.832-4.856(m,1H,NCH),6.834-6.861(m,4H,ArH),7.788(s,1H,Triazole-H),7.967(s , 1H, Triazole-H).
[0062] Other series of compounds with the general formula CAUZJ-A-13-19 were prepared according to the above method. Their compound ...
Embodiment 3
[0068] Embodiment 3, the preparation of compound CAUZJ-A-02 emulsifiable concentrate (5%)
[0069] Add compound CAUZJ-A-045g, Nongru 0203B 15g, penetrant JFC-10.6g into a 100mL volumetric flask, and then dilute to the volume with solvent toluene to obtain 5% emulsifiable concentrate.
[0070] Other emulsifiable concentrates of compounds with the general formula CAUZJ-A can be prepared according to the above method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com