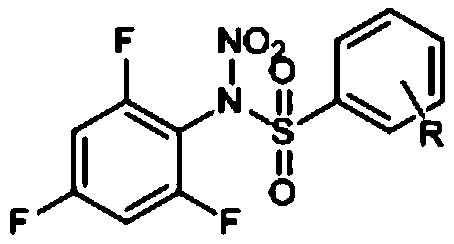
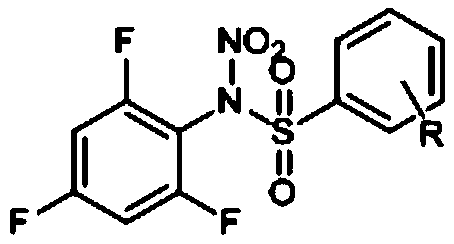
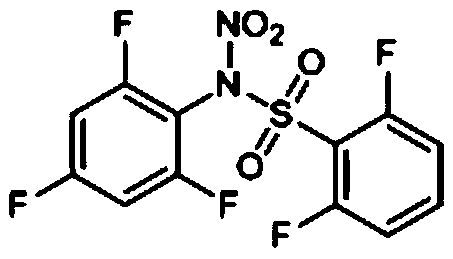
Preparation method and application of N-nitro-N-(2,4,6-trifluorophenyl)benzenesulfonamide compounds
A technology of trifluorophenyl and benzenesulfonamide, which is applied in the field of pesticides, can solve the problems of not obvious bactericidal effect and achieve significant antibacterial activity and good growth regulation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Compound I-1
[0020]
[0021] The synthetic preparation route is as follows:
[0022]
[0023] Add 0.588g (4.0mmol) of 2,4,6-trifluoroaniline into a 50mL three-neck round bottom flask, then add 10mL of chloroform and stir to dissolve, then add 0.64g of pyridine (8.0mmol), and stir for 0.5h , slowly add 0.704g (4.0mmol) 2,6-difluorobenzenesulfonyl chloride dropwise, and stir the reaction at room temperature for 3h. After the reaction is complete, pour 30mL of 5wt% hydrochloric acid into the reaction solution, stir at room temperature for 3min, and then separate the liquids. Take the lower organic layer, mix the sample with silica gel, and purify by column chromatography to obtain the intermediate;
[0024] Add 0.287g of the intermediate (1.0mmol) into a 50mL three-neck round-bottomed flask, then add 6mL of glacial acetic acid and stir to dissolve it, slowly add 3mL of fuming nitric acid dropwise, stir for 30min, then add dropwise 2mL of acetic anhydride, and stir...
Embodiment 2
[0029] By replacing 2,6-difluorobenzenesulfonyl chloride with benzenesulfonyl chloride and referring to the synthesis method of compound I-1, compound I-2 of the present invention can be prepared. The structural formula of compound I-2 is as follows:
[0030]
[0031] Compound 1-2 is a white solid, and its structural characterization data are as follows: 1 H NMR (600MHz, Chloroform-d), δ (ppm): 8.14 (d, J = 7.6Hz, 2H), 7.77 (t, J = 7.5Hz, 1H), 7.64 (t, J = 7.9Hz, 2H) ,6.91(t,J=7.9Hz,2H).
[0032] MS(-ESI)=286.01419(M-NO 2 ).calcd for C 12 h 7 f 3 N 2 o 4 S m / z=332.0786.
Embodiment 3
[0034] By replacing 2,6-difluorobenzenesulfonyl chloride with 4-methylbenzenesulfonyl chloride and referring to the synthesis method of compound I-1, compound I-3 of the present invention can be prepared. The structural formula of compound I-3 is as follows:
[0035]
[0036] Compound I-3 is a white solid, and its structural characterization data are as follows: 1 H NMR (600MHz, Chloroform-d), δ (ppm): 8.01 (d, J = 8.4Hz, 2H), 7.43 (d, J = 8.2Hz, 2H), 6.90 (t, J = 8.0Hz, 2H) ,2.50(s,3H).
[0037] MS(-ESI)=300.02240(M-NO 2 ), calcd.for C 13 h 9 f 3 N 2 o 4 S m / z=346.02351.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com