Method for degrading heparan sulfate and detecting heparan sulfate disaccharide composition
A technology of heparan sulfate and deacetylation, applied in the field of medicine, can solve the problems of poor repeatability of analysis results, cumbersome detection steps, unstable properties, etc., and achieves the effects of low requirements for experimental instruments, short detection time, and small sample consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
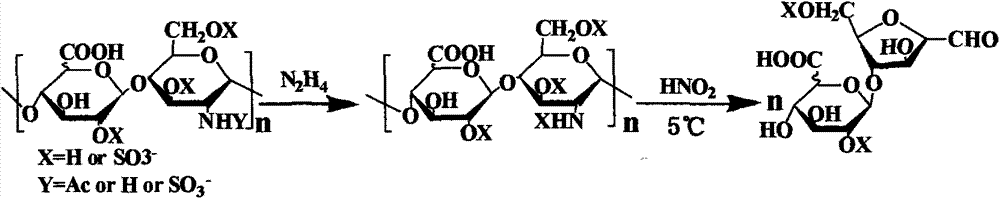
[0035] The chemical degradation of embodiment 1HS
[0036] (1) Dissolve 1mg HS in 500uL containing 10% N 2 h 4 ·H 2 SO 4 N 2 h 4 ·H 2 O solution, heated to dissolve, then sealed and heated to 98 ° C, reacted for 8 hours, after the reaction was completed, freeze-dried to remove N 2 h 4 Deacetylated products are obtained.
[0037] (2) Concentrate the product obtained in step (1) to dryness, dissolve it in 100uL water, add 100uL sodium nitrite aqueous solution with a pH of 1.5, adjust the pH to 4.0 after reacting at 0-5°C for 10 minutes, and add 100uL sodium nitrite with a pH of 4.0 Nitric acid, react at 0-5°C for 10 min, add 60 uL of ammonia water to terminate the reaction, and obtain heparan sulfate disaccharide.
Embodiment 2
[0038] The chemical degradation of embodiment 2HS
[0039] (1) Dissolve 1 mgHS in 1 mL containing 10% N 2 h 4 ·H 2 SO 4 N 2 h 4 ·H 2 O solution, heated to dissolve, then sealed and heated to 90 ° C, reacted for 4 hours, after the reaction was completed, freeze-dried to remove N 2 h 4 Deacetylated products are obtained.
[0040] 2) Concentrate the product obtained in step (1) to dryness, dissolve it in 100uL water, add 100uL sodium nitrite aqueous solution with a pH of 1.5, adjust the pH to 4.0 after reacting at 0-5°C for 10 minutes, and add 100uL sodium nitrite with a pH of 4.0 Nitric acid, react at 0-5°C for 10 minutes, add 60uL of ammonia water to terminate the reaction, and obtain heparan sulfate disaccharide.
Embodiment 3
[0041] Embodiment 3HS chemical degradation
[0042] 1) Dissolve 1mgHS in 1mL containing 10% N 2 h 4 ·H 2 SO 4 N 2 h 4 ·H 2 O solution, heated to dissolve, then sealed and heated to 111 ° C, reacted for 20 hours, after the reaction was completed, freeze-dried to remove N 2 h 4 Deacetylated products are obtained.
[0043] 2) Concentrate the product obtained in step (1) to dryness, dissolve in 100uL of water, add 100uL of sodium nitrite aqueous solution with a pH of 1.5, react at 0-5°C, adjust the pH to 4.0, add 100uL of nitrous acid with a pH of 4.0, React at 0-5°C for 10 minutes, add 60 uL of ammonia water to terminate the reaction, and obtain heparan sulfate disaccharide.
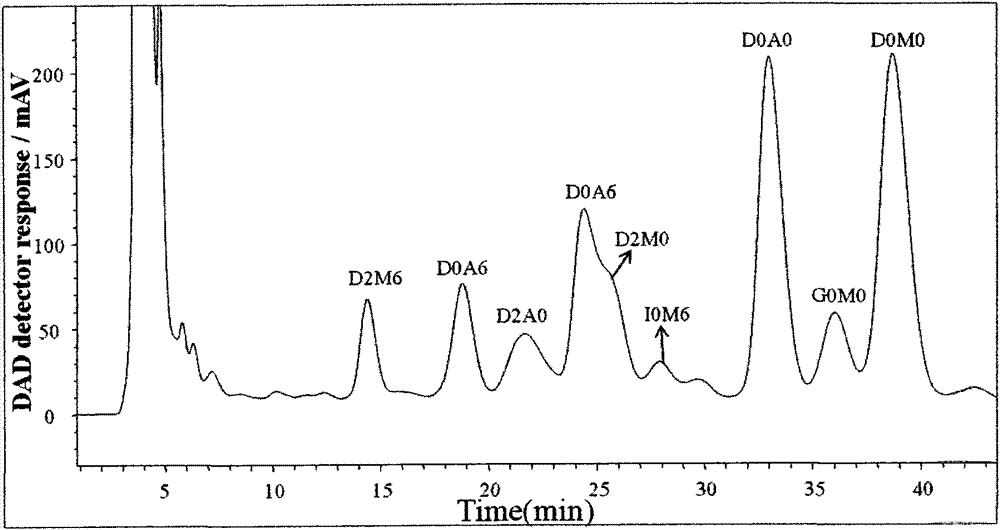
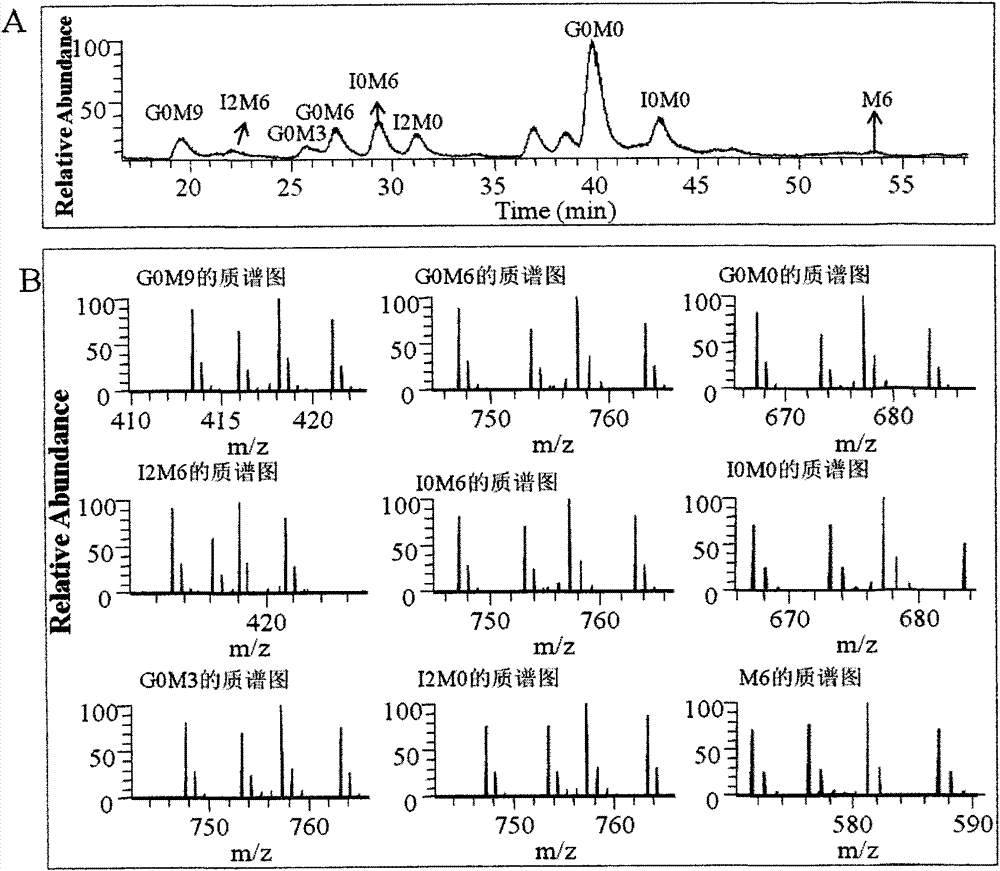
[0044] The degradation products of Examples 1-3 were detected, and the results are shown in Table 1.
[0045] Table 1 Analysis of chemical degradation products
[0046]
[0047]The results show that Examples 1 and 3 can completely chemically degrade HS, and the time used in Example 1 is 60% le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com