Method for reducing content of magnesium in feed grade calcium hydrophosphate
A technology for the content of calcium hydrogen phosphate and magnesium, applied in the field of phosphate, can solve the problems of reducing the content of calcium hydrogen phosphate of feed grade, high cost of nitric acid or nitrate, and great influence of equipment corrosion, and achieves reduction of production cost and simple process. Effective and ensure the effect of stable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
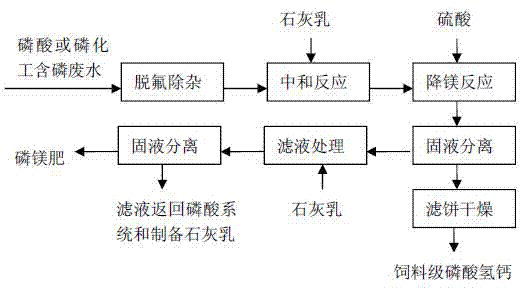
[0019] Take 1t of purification solution (P 2 o 5 is 7%, MgO is 1.1%), add milk of lime and neutralize at 50°C for 1.5h, the pH value at the end of the neutralization reaction is 6.3, at this time, the solid phase in the reaction slurry is about 175kg on a dry basis, of which MgO is about 3.8 %, add 10kg of 98% sulfuric acid and react at 50°C for 25 minutes. The amount of sulfuric acid added is about 0.6 times the stoichiometric ratio of the magnesium content in the solid phase of the slurry at the end of the neutralization reaction. After the reaction, the pH value of the slurry at the end point is 5.2. The slurry after the reaction is centrifuged, and the obtained feed grade calcium hydrogen phosphate product 164kg after the filter cake is dried contains P 2 o 5 39.3%, Ca21.2%, MgO1.9%; the separated liquid phase is neutralized with milk of lime to a pH value of 10, and then filtered, and the filtrate is returned to the phosphoric acid system to prepare milk of lime for rec...
Embodiment 2
[0021] Phosphorus-extracting chemical industry phosphorus-containing wastewater 1t (P 2 o 5 is 6.0%, MgO is 0.62%), adding lime milk and sodium sulfide to defluorinate and remove impurities to obtain a purified solution of about 980kg (P 2 o 5 is 4.6%, MgO is 0.58%), add lime milk and react at 45°C for 1.3h, the pH value at the end of the neutralization reaction is 6.2, at this time, the solid phase in the reaction slurry is about 114kg on a dry basis, of which MgO is about 3.5 %, add 5kg of 98% sulfuric acid and react at 45°C for 30 minutes. The amount of sulfuric acid added is about 0.5 times the stoichiometric ratio of the magnesium content in the solid phase of the slurry at the end of the neutralization reaction. After the reaction, the pH value of the slurry at the end point is 5.5. The slurry after the reaction is centrifuged, and the obtained feed grade calcium hydrogen phosphate product 108kg after the filter cake is dried contains P 2 o 5 39.1%, Ca20.7%, MgO2.0%;...
Embodiment 3
[0023] Phosphorus-extracting chemical industry phosphorus-containing wastewater 1t (P 2 o 5 is 1.6%, MgO is 0.20%), adding lime milk and sodium sulfide to defluorinate and remove impurities to obtain a purified solution of about 990kg (P 2 o 5 1.3% and MgO 0.19%), add milk of lime and neutralize at 40°C for 2 hours, and the pH value at the end of the neutralization reaction is 6.8. At this time, the solid phase in the reaction slurry is about 32kg on a dry basis, of which MgO is about 5.0% , add 7kg of 98% sulfuric acid and react at 35°C for 40 minutes. The amount of sulfuric acid added is about 1.3 times the stoichiometric ratio of the magnesium content in the solid phase of the slurry at the end of the neutralization reaction. After the reaction, the pH value of the slurry at the end is 4.4. The slurry after the reaction is centrifuged, and the obtained feed grade calcium hydrogen phosphate product is 28kg after the filter cake is dried, which contains P 2 o 5 38.7%, Ca2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com