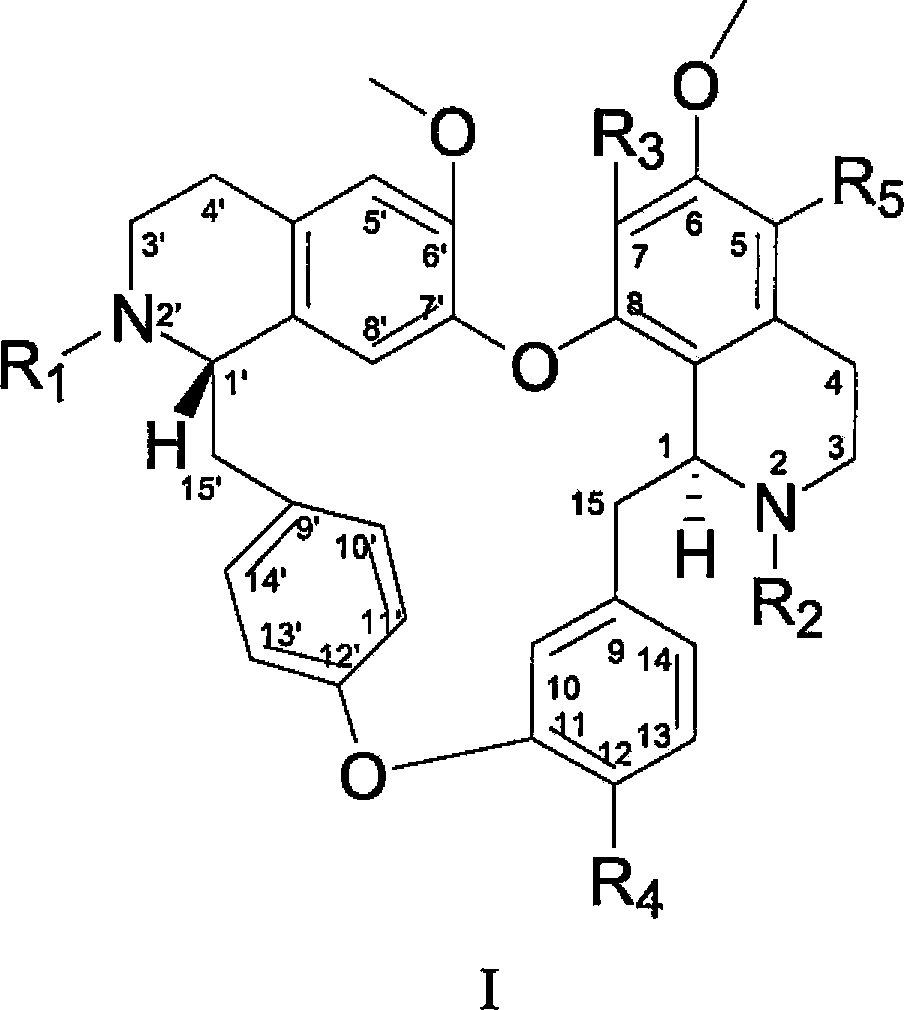
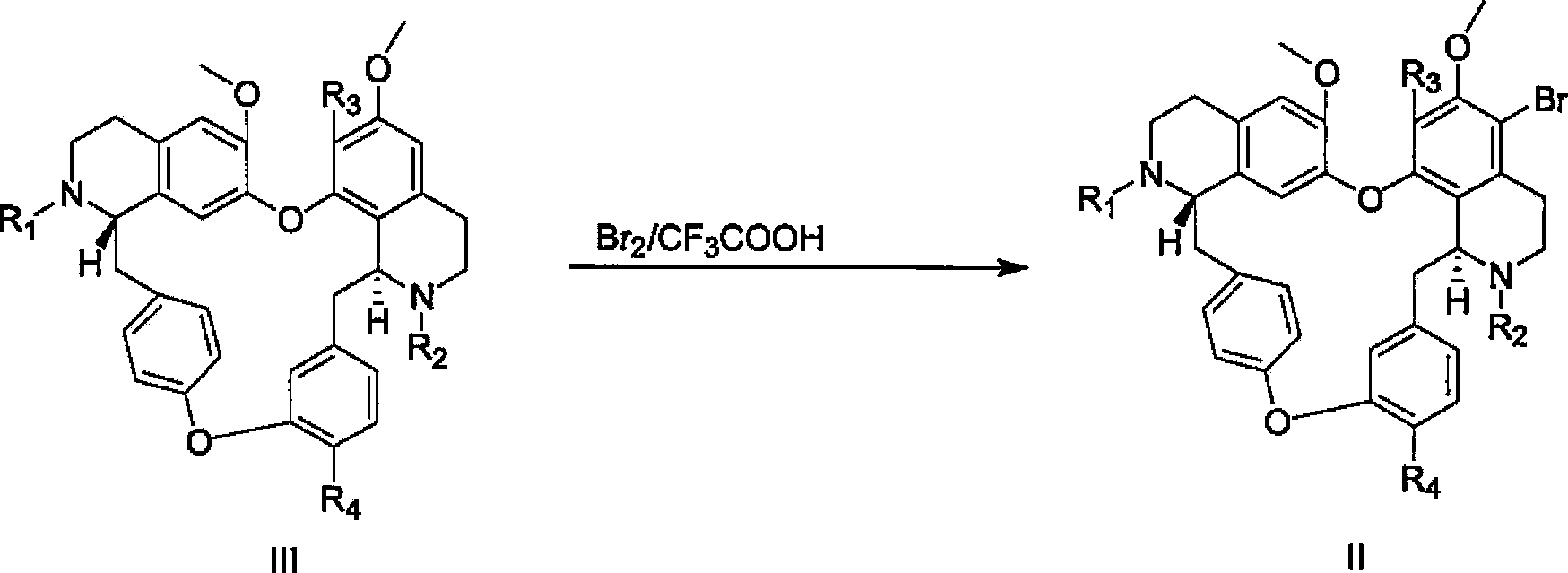Dibenzyl tetrahydroisoquinoline derivative as well as preparation method and application thereof
A technology of alkynyl group and compound, applied in the field of bisbenzyl tetrahydroisoquinoline derivatives and preparation thereof, can solve problems such as not yet seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
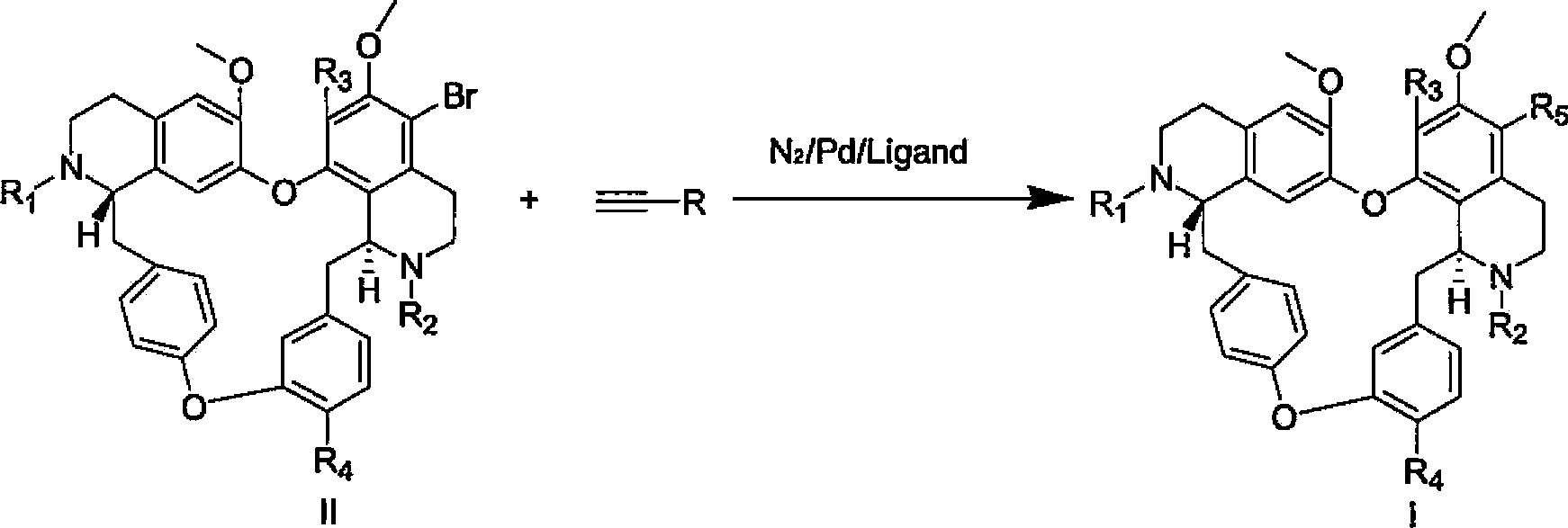
[0050] Example 1: Preparation of 5-(3,3-dimethyl-but-1-ynyl)-tetrandrine
[0051] Weigh 700 mg (1 mmol) of 5-bromo-tetrandrine and dissolve it in 10 ml DMSO, add CsCO 3 651.6mg (2mmol, 2eq), 3,3-dimethyl-1-butyne (CAS: 917-92-0) 164mg (2mmol, 2eq), PdCl 2 (PPh 3 ) 2 21mg (0.03mmol, 0.03eq). Under the protection of nitrogen, the reaction was stirred at 100° C. for 10 hours. After completion of the reaction, filter, add 5ml of ammonia water, then add 30ml of ethyl acetate, wash with saturated brine for 3 times, dry over anhydrous sodium sulfate, perform silica gel column chromatography or ether:methanol (2:1) recrystallization to obtain 5-(3, 3-Dimethyl-but-1-ynyl)-tetrandrine 576 mg. Yield: 82%.
[0052] Properties: Colorless needle crystal.
[0053]
[0054] 5-(3,3-Dimethyl-but-1-ynyl)-tetratridine
[0055] C 44 h 50 N 2 o 6
[0056] MS(ESI):703.35.[M+H] + .
[0057] 1 H NMR (300MHz, CDCl 3 )δ7.32(d, J=8.1Hz, 1H), 7.12(dd, J=8.1, 2.1Hz, 1H), 6.84(m, 2H), 6....
Embodiment 2
[0059] Example 2: Preparation of 5-(hex-1-ynyl)-tetrandrine
[0060] Weigh 700 mg (1 mmol) of 5-bromo-tetrandrine and dissolve it in 10 ml DMSO, add CsCO 3 651.6mg (2mmol, 2eq), 1-hexyne (CAS: 693-02-7) 164mg (2mmol, 2eq), PdCl 2 (PPh 3 ) 2 35mg (0.05mmol, 0.03eq), BINAP20mg (0.03mmol, 0.03eq). Under the protection of nitrogen, the reaction was stirred at 100° C. for 10 hours. After completion of the reaction, filter, add 5ml of ammonia water, then add 30ml of ethyl acetate, wash with saturated brine three times, dry over anhydrous sodium sulfate, perform silica gel column chromatography or ether:methanol (2:1) recrystallization to obtain 5-(hexyl 1-alkynyl)-tetrandrine 527 mg, yield: 75%.
[0061] Properties: white amorphous powder.
[0062]
[0063] 5-(Hex-1-ynyl)-tetrandrine
[0064] C 44 h 50 N 2 o 6
[0065] MS(ESI):703.35.[M+H] + .
[0066] 1 H NMR (300MHz, CDCl 3 )δ7.34(dd, J=8.3, 1.8Hz, 1H), 7.13(dd, J=8.2, 2.5Hz, 1H), 6.86(s, 2H), 6.80(dd, J=8.3, 2....
Embodiment 3
[0068] Example 3: Preparation of 5-(octyl-1,7-diynyl)-tetrandrine
[0069] Weigh 700 mg (1 mmol) of 5-bromo-tetrandrine and dissolve it in 10 ml DMSO, add Ba(OH)2 513mg (3mmol, 2eq), 1,7-octadiyne (CAS: 871-84-1) 212mg (2mmol, 2eq), PdCl 2 (PPh 3 ) 2 35 mg (0.05 mmol, 0.03 eq). Under the protection of nitrogen, the reaction was stirred at 100° C. for 10 hours. After the reaction was completed, filter, add 5ml of ammonia water, then add 30ml of ethyl acetate, wash with saturated brine three times, dry over anhydrous sodium sulfate, perform silica gel column chromatography or ether:methanol (2:1) recrystallization to obtain 5-(octyl- 1,7-diynyl)-tetrandrine 566 mg, yield: 75%.
[0070] Properties: Colorless needle crystal.
[0071]
[0072] 5-(oct-1,7-diynyl)-tetratridine
[0073] C 46 h 50 N 2 o 6
[0074] MS(ESI):727.35.[M+H] + .
[0075] 1 H NMR (300MHz, CDCl 3 )δ7.34(dd, J=8.2, 2.0Hz, 1H), 7.13(dd, J=8.1, 2.5Hz, 1H), 6.85(m, 2H), 6.80(dd, J=8.3, 2.5Hz, 1H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com