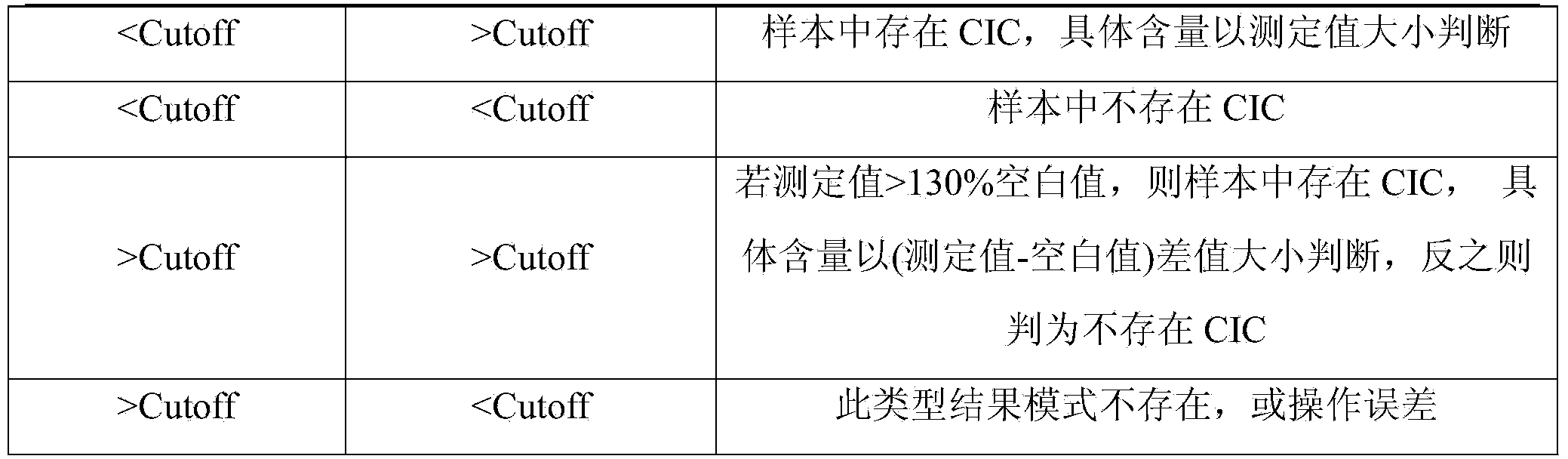
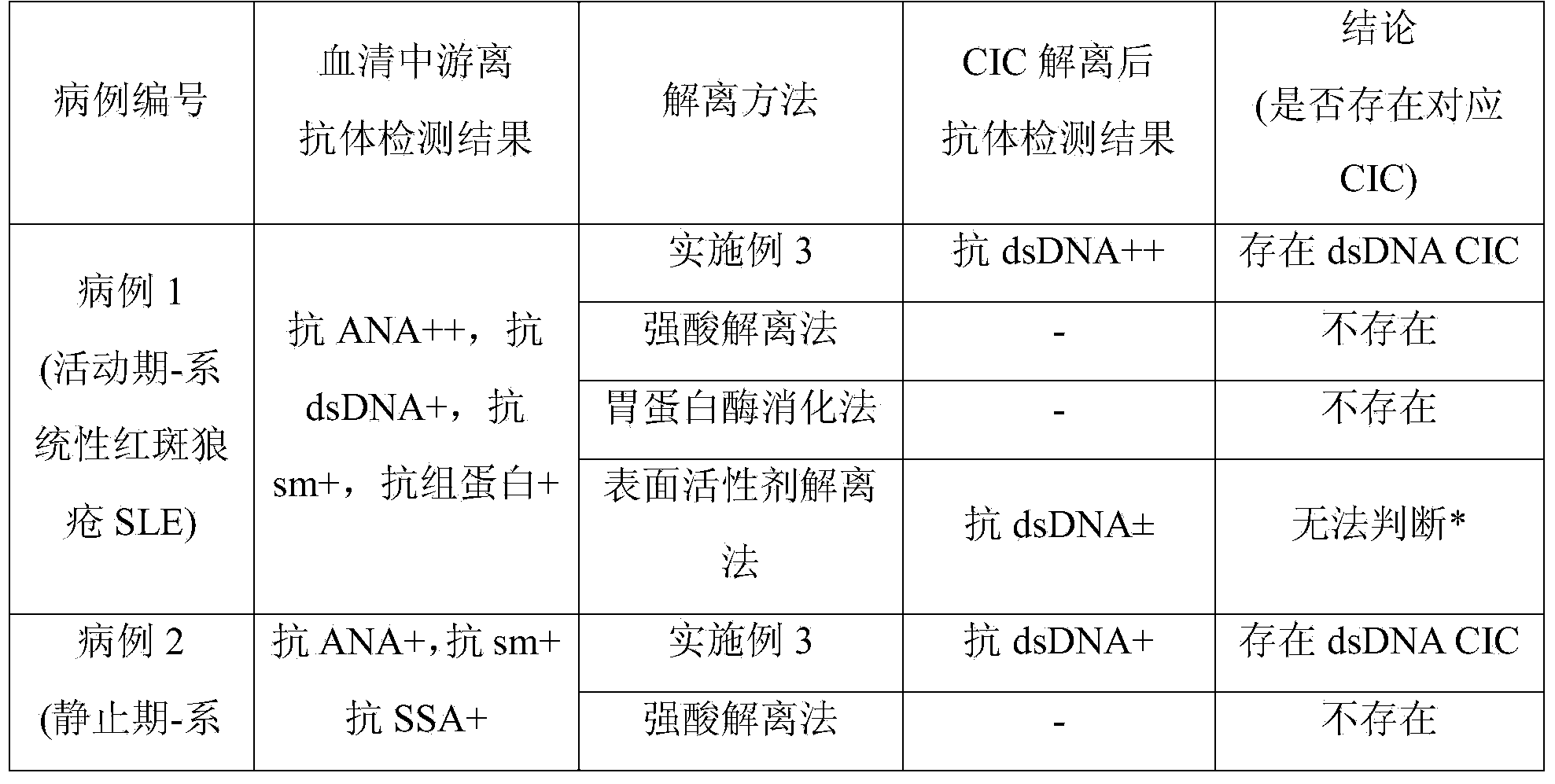
CIC (circulating immune complex) antibody buffer solution dissociation agent for immune complex buffer dissociation agent
A technology of immune complexes and dissociation agents, applied in the field of medical testing, to achieve good repeatability and improve work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] An immune complex buffer dissociation agent, based on the following volume ratio, each component and content are: 135 μl of CIC separation agent, 290 μl of CIC detergent, 5 μl of CIC reconstitution solvent, 65 μl of CIC antibody buffer solution Dissociating agent and 65 μl of CIC antibody buffer to dissociate neutralizing agent.
[0043] Wherein, every 1 L of CIC separation agent includes the following components: 5.2 g of borax, 4.1 g of boric acid, 70 g of polyethylene glycol, 6.2 g of sodium chloride, 100 μl of liquid biological preservative, and distilled water as the balance.
[0044] Each 1 L of CIC detergent includes the following components: 5.2 g of borax, 4.1 g of boric acid, 35 g of polyethylene glycol, 8.0 g of sodium chloride, 100 μl of liquid biological preservative, and distilled water as the balance.
[0045] Each 1 L of CIC reconstitution solvent includes the following components: 8.0 g of sodium chloride, 0.01 g of sodium hydroxide, 50 μl of polyethyle...
Embodiment 2
[0057] An immune complex buffer dissociation agent, based on the following volume ratio, each component and content are: 165 μl of CIC separation agent, 315 μl of CIC detergent, 16 μl of CIC reconstitution solvent, 76 μl of CIC antibody buffer solution Dissociating agent and 76 μl of CIC antibody buffer to dissociate neutralizing agent.
[0058] Wherein, every 1L of CIC separation agent includes the following components: 7.7g of borax, 6.1g of boric acid, 90g of polyethylene glycol, 9.2g of sodium chloride, 500μl of liquid biological preservative, and the balance is distilled water.
[0059] Each 1L of CIC detergent includes the following components: 7.7g of borax, 6.1g of boric acid, 45g of polyethylene glycol, 11.7g of sodium chloride, 500μl of liquid biological preservative, and the balance is distilled water.
[0060] Each 1 L of CIC reconstitution solvent includes the following components: 9.5 g of sodium chloride, 0.03 g of sodium hydroxide, 500 μl of polyethylene glycol...
Embodiment 3
[0072] An immune complex buffer dissociation agent, based on the following volume ratio, each component and content are: 150 μl of CIC separation agent, 300 μl of CIC detergent, 10 μl of CIC reconstitution solvent, 70 μl of CIC antibody buffer solution Dissociating agent and 70 μl of CIC antibody buffer to dissociate neutralizing agent.
[0073] Wherein, every 1L of CIC separation agent includes the following components: 6.4g of borax, 5.1g of boric acid, 80g of polyethylene glycol, 7.7g of sodium chloride, 200μl of liquid biological preservative, and the balance is distilled water.
[0074] Each 1L of CIC detergent includes the following components: 6.4g of borax, 5.1g of boric acid, 40g of polyethylene glycol, 9.9g of sodium chloride, 200μl of liquid biological preservative, and the balance is distilled water.
[0075] Each 1 L of CIC reconstitution solvent includes the following components: 9.0 g of sodium chloride, 0.016 g of sodium hydroxide, 300 μl of polyethylene glycol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com