Fosfluconazole lyophilized preparation composition stable at normal temperature and preparation method thereof
A technology of fosfluconazole and freeze-dried preparations, applied in freeze-dried transportation, antifungal agents, powder transportation, etc., can solve problems such as poor stability, increased production costs, unfavorable storage for hospitals and patients, and achieve improved stability , Conducive to the effect of convenient production, storage and portability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
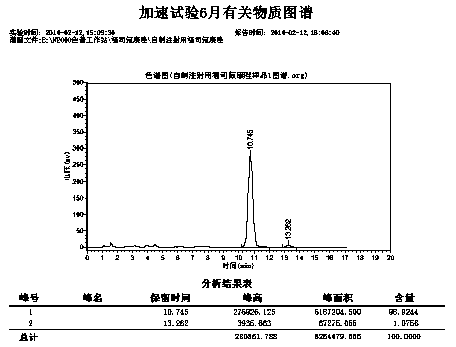
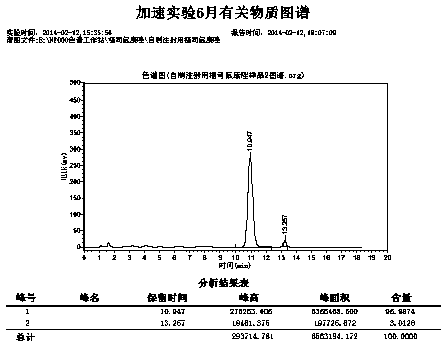
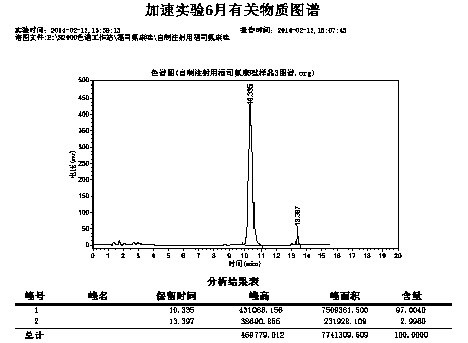
Image
Examples
Embodiment 1
[0028] Prescription:
[0029]
[0030] Preparation Process:
[0031] Take about 90% of the total amount of water for injection, control the water temperature between 20°C and 25°C, add 302.7g of injection-grade arginine and 504.5g of fosfluconazole, which are accurately weighed, stir well until they are completely dissolved, and measure the pH value. At this time, the pH value is 8.9, add water for injection to the full amount, add 0.1% activated carbon for needles, stir for 30 minutes at 20 ° C ~ 25 ° C, filter with a 0.22 μm microporous membrane to sterilize, and divide the solution into 10 ml vials , the volume of each bottle is 5ml.
[0032] The prepared cillin bottle filled with fosfluconazole is put into the freeze-drying box, and then the temperature in the freeze-drying machine is reduced to below -45°C within 2 hours to make it freeze rapidly. Vacuumize and make the atmospheric pressure in the box reach 2.66pa within 30 minutes. Dry according to the temperature...
Embodiment 2
[0034] Prescription:
[0035]
[0036] Preparation Process:
[0037] Take about 90% of the total amount of water for injection in the prescription. The water temperature is controlled at 20°C to 25°C. Add 152.4g of injection-grade arginine and 252.3g of fosfluconazole, which are precisely weighed, and stir until they are completely dissolved. Measure the pH value. At this time, the pH value is 9.0, add water for injection to the full amount, add 0.1% activated carbon for needles, stir for 30 minutes at 20°C to 25°C, filter with a 0.22μm microporous filter membrane, and pack the solution into 7ml vials , the volume of each bottle is 2.5ml.
[0038] Put the prepared cillin bottle filled with fosfluconazole into the freeze-drying box, and then reduce the temperature inside the freeze-drying machine to below -45°C within 2 hours to make it freeze quickly. Vacuumize and make the atmospheric pressure in the box reach 2.66pa within 30 minutes. Dry according to the temperature ...
Embodiment 3
[0040] Prescription:
[0041]
[0042] Preparation Process:
[0043] Take about 90% of the total amount of water for injection, and control the water temperature at 20°C to 25°C, add 302.7g of injection-grade arginine, 504.5g of fosfluconazole and 1009g of injection-grade mannitol, and stir well until completely dissolved , measure the pH value, at this time the pH value is 9.1, add water for injection to the full amount, add 0.1% activated carbon for needles, stir for 30 minutes at 20 ° C ~ 25 ° C, sterilize and filter the solution with a 0.22 μm microporous membrane, and pack the solution in 10 ml specifications In the vials, the volume of each bottle is 5ml.
[0044]The prepared cillin bottle filled with fosfluconazole is put into the freeze-drying box, and then the temperature in the freeze-drying machine is reduced to below -45°C within 2 hours to make it freeze rapidly. Vacuumize and make the atmospheric pressure in the box reach 2.66pa within 30 minutes. Dry acco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com