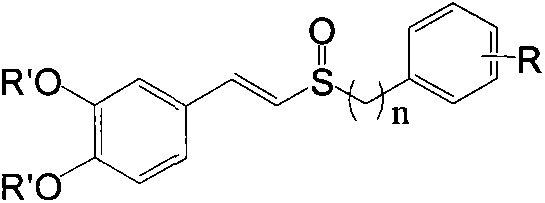
Preparation method of E-3,4-dihydroxyphenylvinyl sulfoxide and application of E-3,4-dihydroxyphenylvinyl sulfoxide as nerve protection drug
A technology of dihydroxyphenyl and hydroxyl protecting groups is applied in the preparation of E-3,4-dihydroxystyryl sulfoxide compounds and its application field as a neuroprotective drug, and can solve the problem that the blood-brain barrier is not easily penetrated. , fast metabolism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
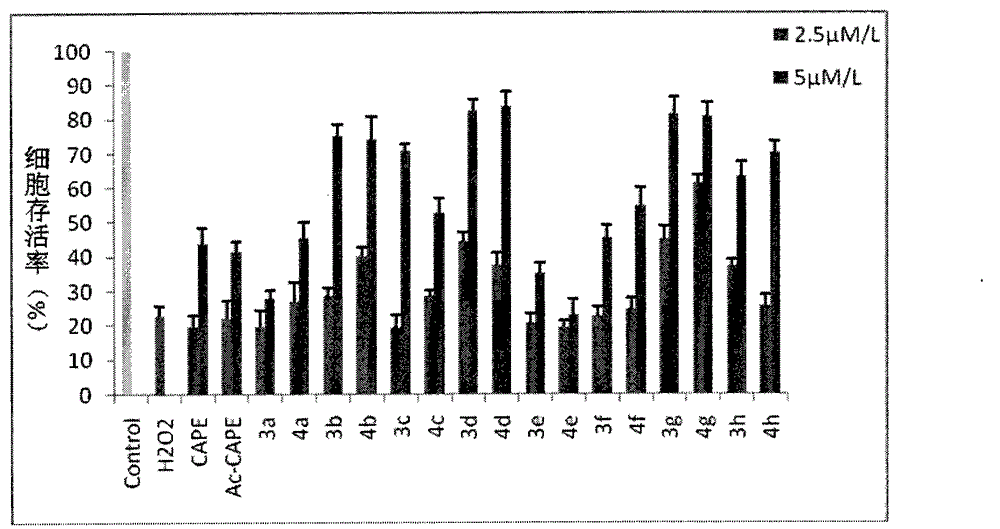
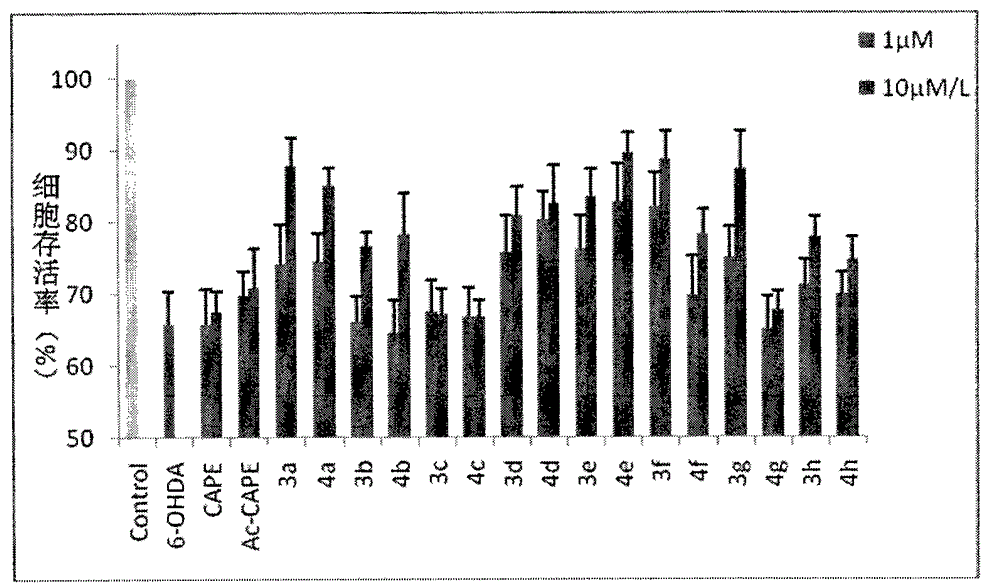
Image
Examples
Embodiment 1
[0032] Preparation of 2-(4-chloro)benzylthioacetic acid (1b)
[0033] Using the synthesis method of compound 1a above, 4-chlorobenzyl bromide was used as a reactant to obtain white solid 1b with a yield of 85.5%, m.p.51-52°C. 1 H NMR (400MHz, DMSO-d 6 )δ=12.62(s, 1H, COOH), 7.32-7.50(m, 4H, ArH), 3.82(s, 2H, SCH 2 COOH), 3.14(s, 2H, ArCH 2 S)
[0034] Preparation of 2-(4-tert-butyl)benzylthioacetic acid (1c)
[0035] Using the synthesis method of compound 1a above, 4-tert-butylbenzyl bromide was used as a reactant to obtain white solid 1c with a yield of 73.9%, m.p.75-76°C. 1 H NMR (400MHz, DMSO-d 6 )δ=12.58(s, 1H, COOH), 7.22-7.36(m, 4H, ArH), 3.77(s, 2H, SCH 2 COOH), 3.12(s, 2H, ArCH 2 S), 1.27(s, 9H, C(CH 3 ) 3 ).
[0036] Preparation of 2-(4-trifluoromethyl)benzylthioacetic acid (1d)
[0037] Using the synthesis method of compound 1a above, using 4-trifluoromethylbenzyl bromide as a reactant, a white solid 1d was obtained with a yield of 88.0%, m.p.64-65°C. 1 H...
Embodiment 2
[0049] Preparation of 2-(4-chloro)benzylsulfinylacetic acid (2b)
[0050] Using the synthesis method of compound 2a above, 2-(4-chloro)benzylthioacetic acid was used as a reactant to obtain white solid 2b with a yield of 82.0%, m.p.146-147°C. 1 H NMR (400MHz, DMSO-d 6 )δ=13.14(s, 1H, COOH), 7.31-7.44(m, 4H, ArH), 4.22(d, J=12.4Hz, 1H, SCH 2 COOH), 4.05 (d, J=12.4Hz, 1H, SCH 2 COOH), 3.83 (d, J=14.8Hz, 1H, ArCH 2 SO), 3.50 (d, J=14.8Hz, 1H, ArCH 2 SO)
[0051] Preparation of 2-(4-tert-butyl)benzylsulfinylacetic acid (2c)
[0052]Using the synthesis method of compound 2a above, 2-(4-tert-butyl)benzylthioacetic acid was used as the reactant to obtain white solid 2c with a yield of 73.8%, m.p.145-146°C. 1 H NMR (400MHz, DMSO-d 6 )δ=13.14(s, 1H, COOH), 7.24-7.42(m, 4H, ArH), 4.21(d, J=12.8Hz, 1H, SCH 2 COOH), 4.02 (d, J=12.8Hz, 1H, SCH 2 COOH), 3.86 (d, J=14.4Hz, 1H, ArCH 2 SO), 3.56 (d, J=14.4Hz, 1H, ArCH 2 SO), 1.29(s, 9H, C(CH 3 ) 3 )
[0053] Preparation of 2-(4-t...
Embodiment 3
[0067] Using the synthesis method of compound 2a above, 2-(4-chloro)benzylthioacetic acid was used as a reactant to obtain white solid 2b with a yield of 70.0%.
[0068] Example 4:
[0069] Preparation of 2-benzylsulfinylacetic acid (2a)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com