Method for synthesizing 2,6-naphthalene dicarboxylic acid
A technology for naphthalene dicarboxylic acid and diisopropylnaphthalene, applied in the field of preparing 2,6-naphthalene dicarboxylic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
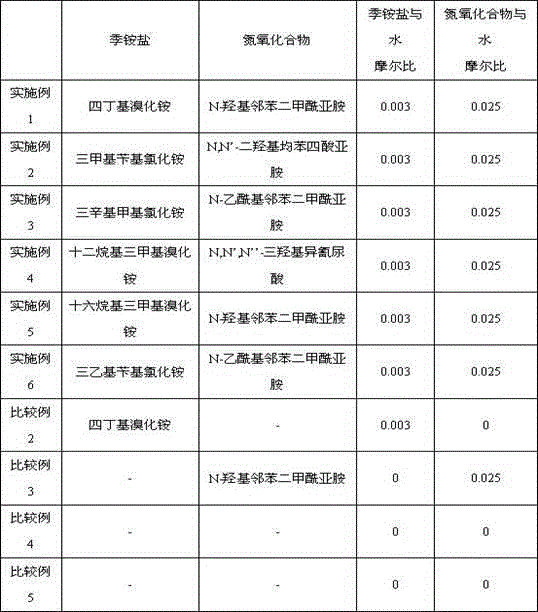
Embodiment 1
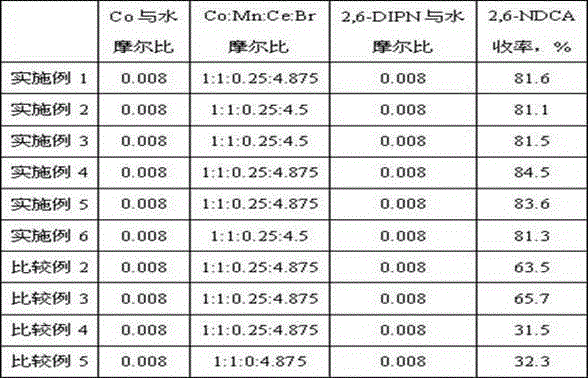
[0013] Preparation of 2,6-naphthalene dicarboxylic acid: 2.5mol water, 0.02molCoBr 2 , 0.02mol MnBr 2 , 0.005CeBr 3 , 0.005mol potassium bromide, 0.0075mol tetrabutylammonium bromide, 0.0625mol N-hydroxyphthalimide (NHPI) and 0.02mol 2,6-diisopropylnaphthalene were added to the titanium reactor, first with After the nitrogen is discharged from the kettle, pressurize to 2.5MPa, increase the stirring speed to 800rpm, continuously supply compressed air to the reaction pressure of 3.0MPa, and at the same time stir and heat up to the reaction temperature, control the reaction temperature to 200°C, and the reaction pressure to 3.0MPa. The molar ratio of 2,6-diisopropylnaphthalene was 40, and the reaction was stopped after continuing the reaction for 3 hours.
[0014] Product analysis: The reaction mixture obtained from the above reaction was cooled and filtered, and the obtained solid was washed with hot distilled water at 80° C., and then dried at 85° C. for 5 hours to obtain the...
Embodiment 2-6
[0016] The types of quaternary ammonium salts and nitrogen oxides were changed without changing the molar amount of them, and the rest of the operations were the same as in Example 1. The yields of 2,6-naphthalene dicarboxylic acid obtained in each example are shown in Table 1.
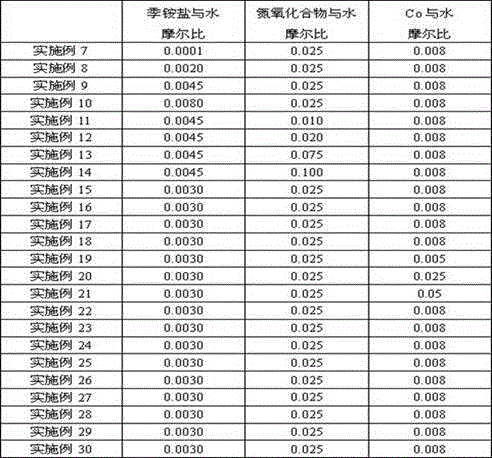
Embodiment 7-14
[0018] Change the input amount of tetrabutylammonium bromide and N-hydroxyphthalimide (NHPI), do not change the input amount of other raw materials, and the rest of the operations are the same as in Example 1. The 2,6-naphthalene obtained in each embodiment The yields of diformic acid are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com