Polymetallic oxygen catalyst for degradation of unsymmetrical dimethylhydrazine and its preparation method and application
A technology of polymetallic oxygen and unsymmetrical dimethylhydrazine, applied in the direction of metal/metal oxide/metal hydroxide catalyst, molecular sieve catalyst, chemical instrument and method, etc., can solve the problem that is not suitable for large-scale treatment of high-concentration unsymmetrical dimethylhydrazine Sewage, ultraviolet photons cannot be fully used for the reaction, high-concentration unsymmetrical dimethylhydrazine is not fully degraded, etc., to achieve good industrial application prospects, small secondary pollution, and improve activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
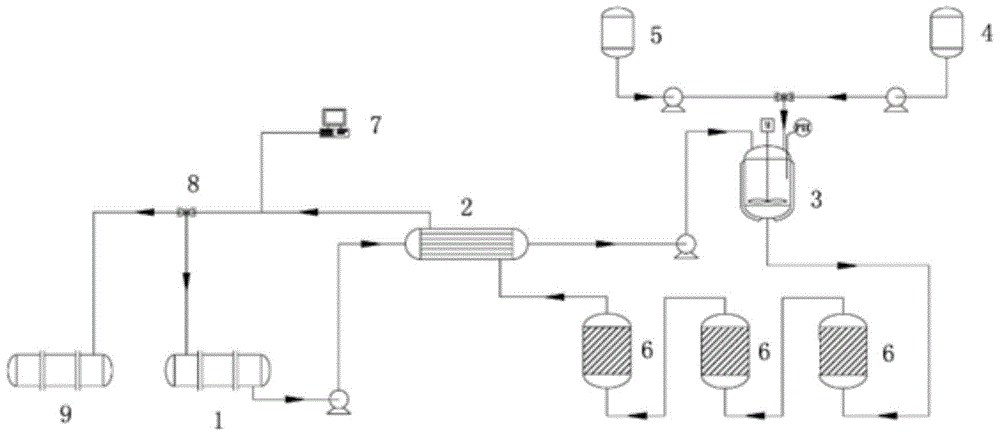
[0032] Secondly, the preparation method of the above-mentioned polymetallic oxygen group catalyst is a precipitation-oxidation method, which mainly includes the following steps: using at least two metals in transition metals and / or rare earth metals, such as copper nickel cerium, copper zinc zirconium, nickel zirconium, zinc Lanthanum or copper-nickel as the active ingredient, adding alkaline precipitation aids such as (NH 4 ) 2 C 2 o 4 Or hydrazine hydrate, adjust the pH value, for example, it can be controlled between 5-7, adjust the temperature, for example, it can be controlled between 40-70 °C, and then post-load it on the natural porous carrier, such as zeolite powder, molecular sieve powder, silicon Alite, montmorillonite or kaolin, after oxidizing agent such as KMnO 4 Oxidation and calcination, the calcination temperature can be controlled at 350-600°C to prepare a multi-metal oxygen group catalyst. Wherein, the weight ratio of the metal oxide to the natural porous...
Embodiment 1
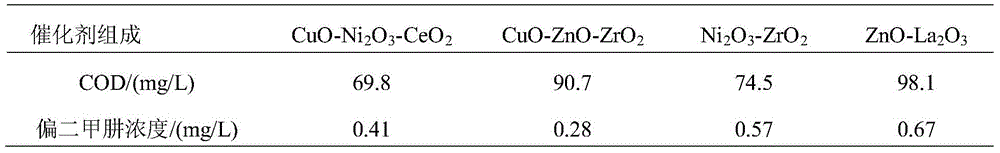
[0034] Embodiment 1 Catalyst active center composition is on the influence of degradation reaction
[0035]Weigh at least two combinations of metal cations that are copper, nickel, cerium, zinc, zirconium, and lanthanum salt compounds, dissolve them in 500mL distilled aqueous solution in a water bath at 70°C, add hydrazine hydrate solution dropwise after dissolution, and add carrier zeolite Powder 100g, add acid and alkali to control the pH value to 7, stir for 6h. Then add 3.6g potassium permanganate and stir for 10h. Filter, wash with water, dry the solid product at 110°C, and place it in a muffle furnace for roasting at 400°C for 3h. The following four kinds of multi-metal oxide catalysts with different active center compositions were prepared: CuO-Ni 2 o 3 -CeO 2 –MnO x – Zeolite, CuO-ZnO-ZrO 2 –MnO x – Zeolite, Ni 2 o 3 -ZrO 2 –MnO x – Zeolite, ZnO-La 2 o 3 –MnO x - Zeolite, wherein the weight of the metal oxide and the support in the four catalysts is contr...
Embodiment 2
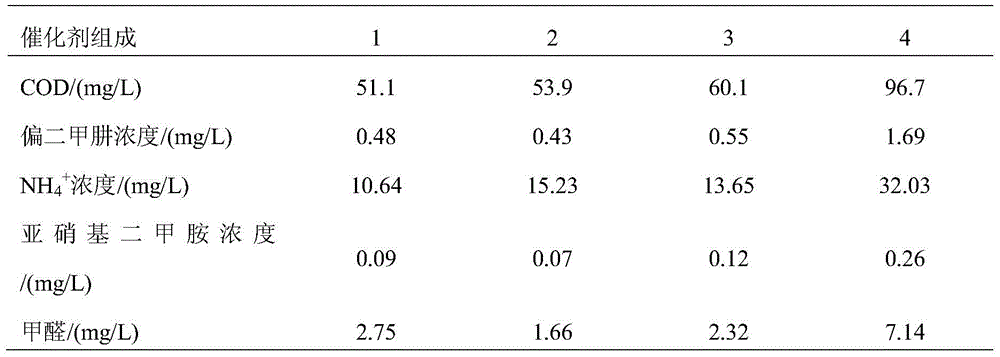
[0040] Embodiment 2 The influence of catalyst active center composition ratio on degradation reaction
[0041] Catalyst composition 1, add copper nitrate (Cu(NO 3 ) 2 ·3H 2 O), nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O), after it dissolves, add dropwise (NH 4 ) 2 C 2 o 4 Solution, 80g of diatomaceous earth, add acid and alkali to control the pH value to 6, and stir for 6h. Then add 5.4g potassium permanganate and stir for 10h. Filter, wash with water, dry the solid product at 110°C, and place it in a muffle furnace for roasting at 400°C for 4h. In the catalyst obtained after firing, the weight ratio of copper oxide to carrier diatomite is 1.0:100, and the weight ratio of nickel oxide to carrier diatomite is 1.0:100. MnO 2 Gauge, MnO 2 The weight ratio to the carrier diatomaceous earth is 3.0:100.
[0042] Catalyst composition 2, add copper nitrate (Cu(NO 3 ) 2 ·3H 2 O), nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O), after it dissolves, add dropwise (NH 4 ) 2 C 2 o 4 S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com