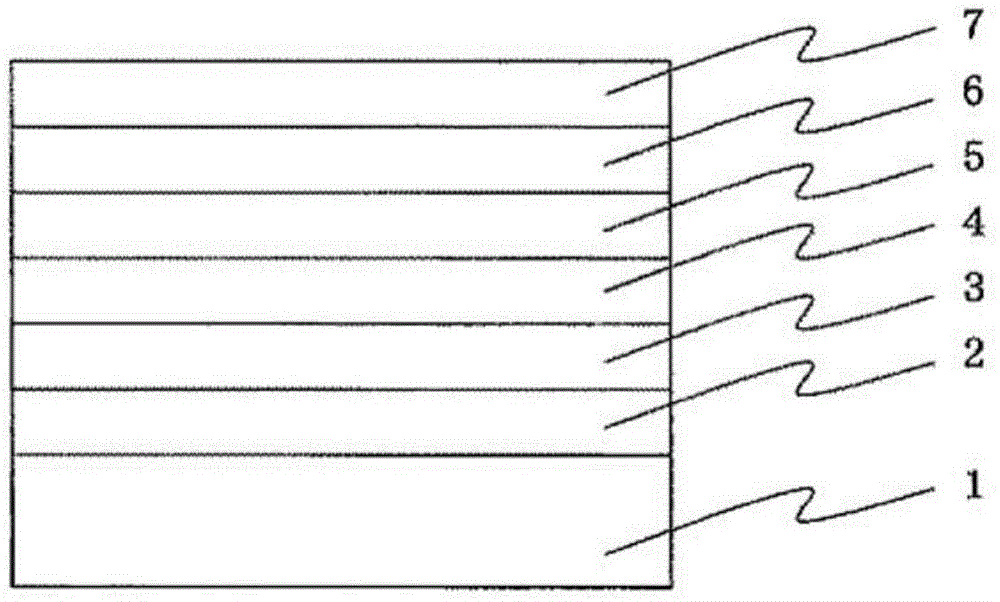
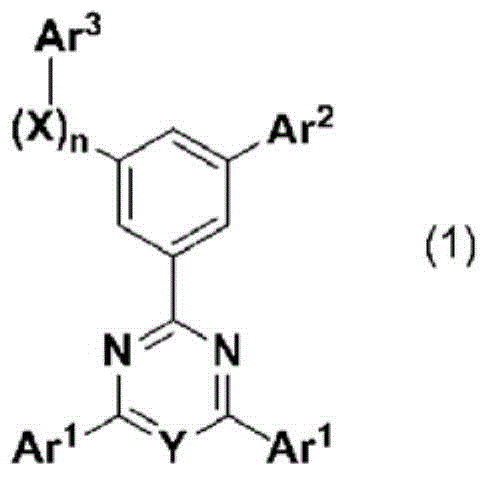
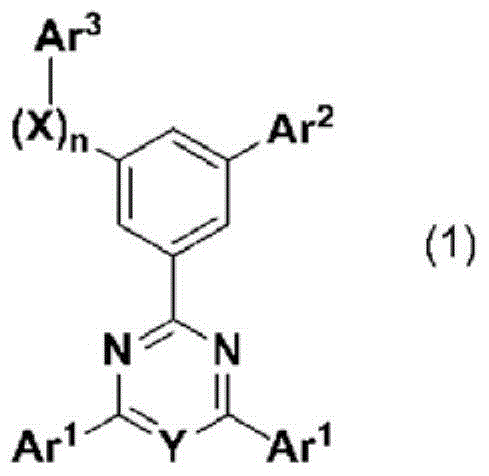
Cyclic azine compound having nitrogen-containing fused aromatic group, method for producing same, and organic electroluminescent element using same as constituent component
一种环状吖嗪、化合物的技术,应用在高效率有机电致发光元件领域,能够解决没有记载化合物实施例等问题,达到驱动性及发光性优异、良好电荷注入及传输特性、寿命长的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0166] Hereinafter, examples and test examples are given to describe the present invention in more detail, but the present invention is not limited to these examples.
[0167] Reference example-1
[0168]
[0169] 3,5-Dibromobenzoyl chloride (5.97 g) and benzonitrile (4.12 g) were dissolved in chloroform (50 mL), and after cooling to 0° C., antimony pentachloride (5.98 g) was added dropwise. After the mixture was stirred at room temperature for 10 minutes, it was refluxed for 22 hours. After cooling the reaction mixture to room temperature, chloroform was distilled off under reduced pressure to obtain a yellow solid.
[0170] When the obtained yellow solid was added to 28% aqueous ammonia solution (300 mL) cooled to 0° C., a white solid was formed. After stirring at room temperature for 1 hour and filtering, the obtained white solid was washed with water and methanol. The resulting white solid was purified by silica gel column chromatography to obtain a white solid of 2-...
reference example -2
[0174]
[0175] Under argon flow, take 3,5-dibromobenzoyl chloride (29.8g) and p-toluonitrile (23.4g) in a 500mL three-port reaction vessel equipped with a reflux tube and a mechanical stirrer, add chlorobenzene (200mL) and dissolve . The obtained solution was cooled to 0° C., and antimony pentachloride (29.9 g) was added dropwise. The mixture was refluxed at room temperature for 1 hour, and further refluxed at 100°C for 2 hours. The resulting dark red suspension was cooled to -20°C and 28% aqueous ammonia solution (135 mL) was added. After stirring this milky white suspension at room temperature for 30 minutes, it heated slowly to 140 degreeC using an oil bath, and distilled off a solvent. Chlorobenzene (100 mL) was added, heated at 130° C., and filtered to remove insoluble matter. After cooling the filtrate naturally, methanol (100 mL) was added. The precipitated solid was filtered off, washed with methanol (30 mL×2) and dried to obtain the target product 2-(3,5-dibro...
Embodiment -1
[0207]
[0208] 2-Chloro-1,10-phenanthroline (4.26g), 2-[3,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxo Borolan-2-yl)phenyl]-4,6-diphenyl-1,3,5-triazine (4.67g), lithium chloride (1.06g) and tetrakis(triphenylphosphine)palladium ( 767mg) was suspended in a mixed solvent of toluene (200mL) and ethanol (50mL), 2.0M aqueous sodium carbonate solution (33.2mL) was added, and stirred at 100°C for 94 hours. After natural cooling, the low-boiling components were distilled off under reduced pressure, and the residue was purified by alumina column chromatography (developing solvent: hexane: chloroform = 1:2 to 0:1), and then recovered from a mixed solvent of dichloromethane and methanol. Crystallization, thus obtaining the target 2-[3,5-bis(1,10-phenanthrolin-2-yl)phenyl]-4,6-diphenyl-1,3,5-triazine white powder (yield 4.76g, yield 86%).
[0209] 1 H-NMR (CDCl 3 ): δ7.64-7.66 (m, 6H), 7.69 (dd, J = 4.3, 8.0Hz, 2H), 7.85 (d, J = 8.8Hz, 2H), 7.91 (d, J = 8.8Hz, 2H) , 8.31(dd, J=1.7, 8.0H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com