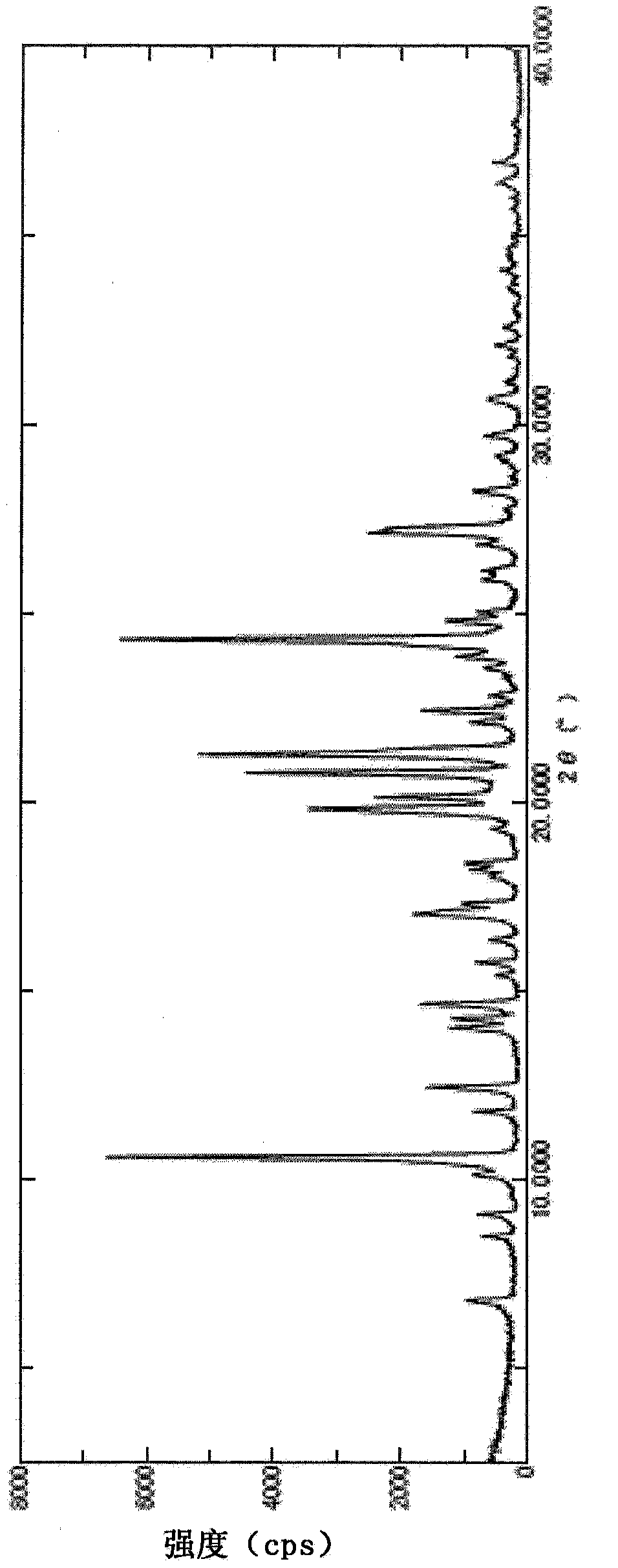
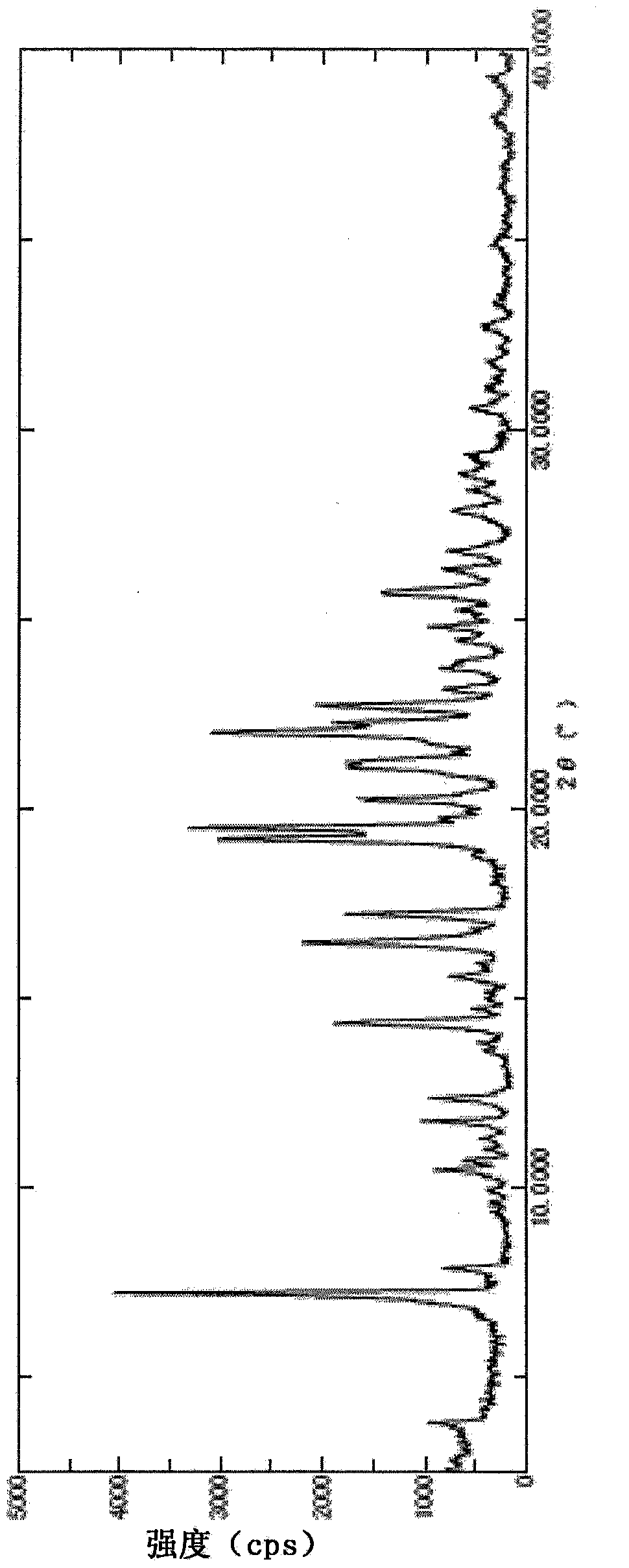
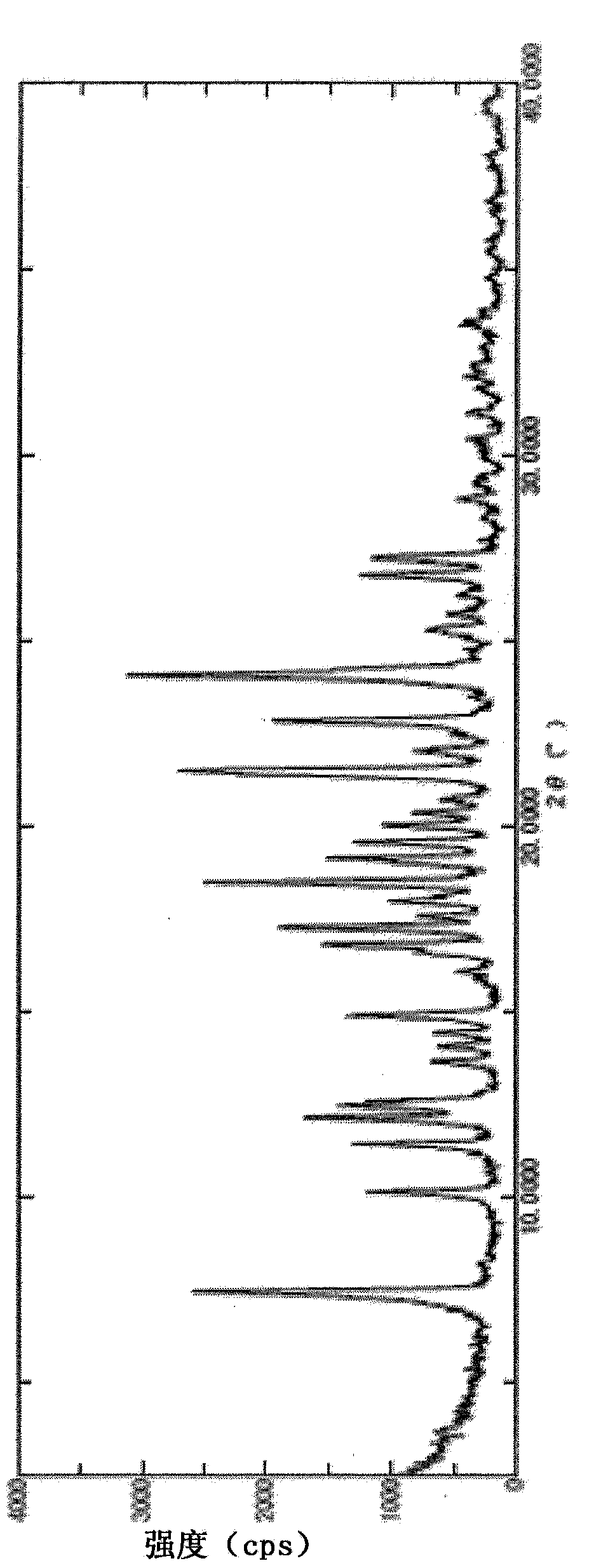
Pyrazine carboxamide compound
A technology of compound and formamide, applied in the field of pharmaceutical composition, can solve problems such as undisclosed effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0351] Hereinafter, the production method of the compound of formula (I) is demonstrated in more detail based on an Example. It should be noted that the present invention is not limited to the compounds described in the following examples. In addition, the production methods of the raw material compounds are shown in the production examples, respectively. In addition, the production method of the compound of formula (I) is not limited to the production method of the specific examples shown below, and the compound of formula (I) can also be produced by a combination of these production methods or methods obvious to those skilled in the art. to manufacture.
[0352] In addition, the following abbreviations are sometimes used in Examples, Production Examples, and Tables described below.
[0353] PEx: production example number, Ex: example number, PSyn: production example number produced by the same method, Syn: example number produced by the same method (for example, E1 represe...
manufacture example 1
[0358] 3-nitrophenol (1g), 3,5-dichloro-6-ethylpyrazine-2-carboxamide (1.74g), diisopropylethylamine (2.63mL), di The mixture of alkanes (10 mL) was stirred overnight at 80°C. Water was added to the reaction mixture, and the precipitated solid was collected by filtration and dried under reduced pressure to obtain 3-chloro-6-ethyl-5-(3-nitrophenoxy)pyrazine-2 as a white solid. - Formamide (1.68 g).
manufacture example 2
[0360] 3-Chloro-6-ethyl-5-(3-nitrophenoxy)pyrazine-2-carboxamide (500mg), 4-(4-methylpiperazin-1-yl)aniline (300mg) , methanesulfonic acid (201 μL), and N-methylpyrrolidone (2 mL) were heated at 200° C. for 1 hour using a microwave reactor. 4-(4-Methylpiperazin-1-yl)aniline (150 mg) was added to the reaction mixture, followed by heating at 200°C for 30 minutes. A saturated aqueous sodium bicarbonate solution was added to the reaction mixture, and the precipitated solid was collected by filtration and dried. The obtained solid was purified by silica gel column chromatography (eluent; chloroform:methanol:28% ammonia water=1:0:0-200:10:1) to obtain 6-ethyl-3 -{[4-(4-Methylpiperazin-1-yl)phenyl]amino}-5-(3-nitrophenoxy)pyrazine-2-carboxamide (308 mg).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com