A kind of Lansoprazole enteric-coated tablet and preparation method thereof
A technology for lansoprazole enteric and lansoprazole, which is applied to the field of lansoprazole enteric-coated tablets and the preparation thereof, can solve the problems of unstable process quality control and the like, achieves improved oral bioavailability, good release condition, The effect of improving solubility and release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
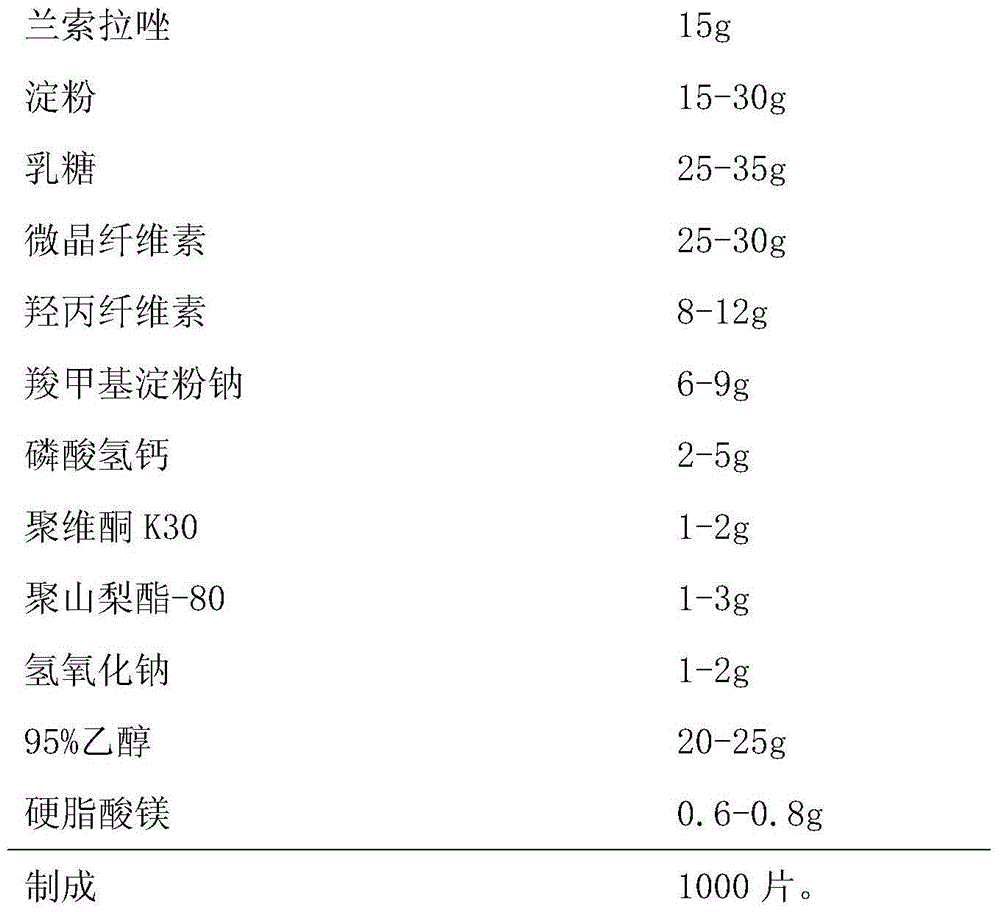
[0030] Embodiment 1, a kind of Lansoprazole enteric-coated tablet, the composition of every 1000 is as follows,
[0031] Chip:
[0032]
[0033] Isolation layer coating: hydroxypropyl methylcellulose ethanol solution (containing 1% HPMC, 3% talc, 3% PEG 6000, 1% Tween 280, 1% diethyl phthalate, 80% 95% ethanol );
[0034] Enteric layer coating: polyacrylic acid resin EudragitL100-55 aqueous suspension (containing 13% EudragitL100-55, 0.1% sodium hydroxide, 2% PEG6000, 3.4% talc, 1.5% titanium dioxide)
[0035] Above-mentioned lansoprazole enteric-coated tablet preparation method is:
[0036] (1) Take by weighing a sufficient amount of starch, lactose, microcrystalline cellulose, hydroxypropyl cellulose, calcium hydrogen phosphate and 6g sodium carboxymethyl starch according to the prescription quantity and mix;
[0037] (2) Measure 95% ethanol, add the sodium hydroxide of prescription quantity, stir and make dissolving; Add the povidone K30 of prescription quantity, stir...
Embodiment 2
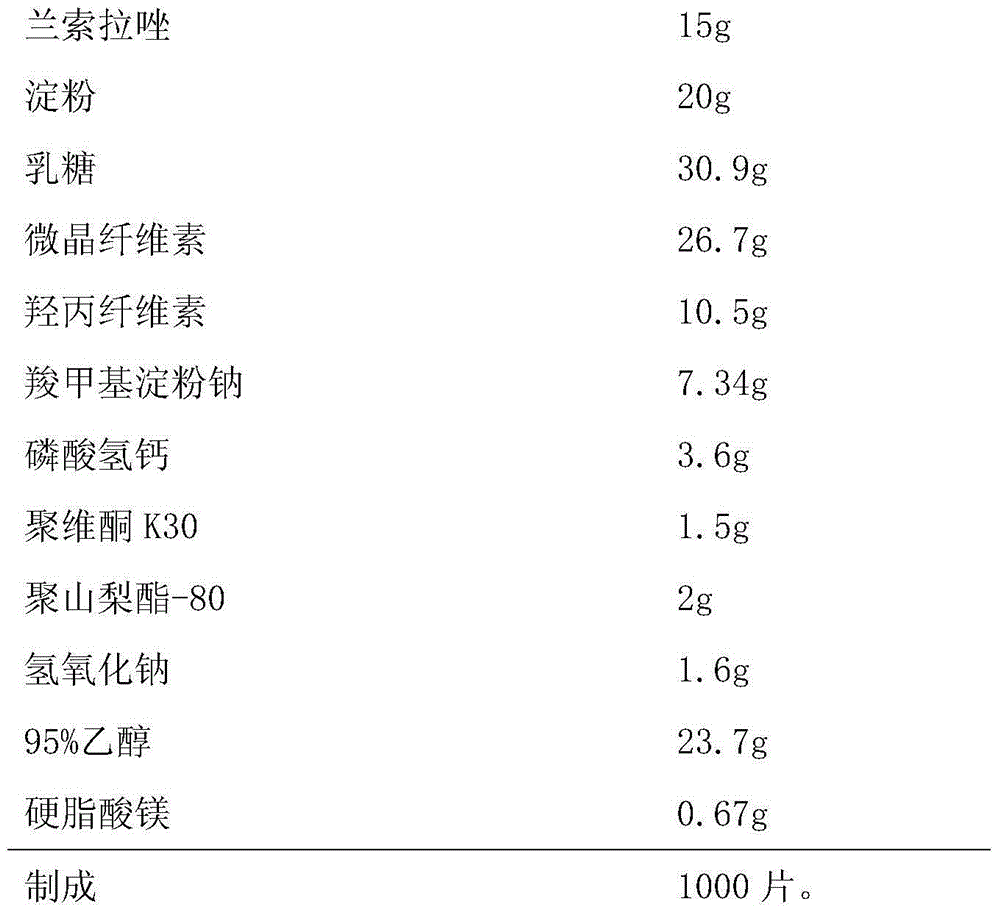
[0044] Embodiment 2, a kind of Lansoprazole enteric-coated tablet, the composition of every 1000 is as follows,
[0045] Chip:
[0046]
[0047] Isolation layer coating: hypromellose ethanol solution (containing 1% HPMC, 3% talc, 3% PEG 6000, 1% Tween 280, 1% diethyl phthalate, 80% 95% ethanol );
[0048] Enteric layer coating: polyacrylic acid resin EudragitL100-55 aqueous suspension (containing 13% EudragitL100-55, 0.1% sodium hydroxide, 2% PEG6000, 3.4% talc, 1.5% titanium dioxide)
[0049] Above-mentioned lansoprazole enteric-coated tablet preparation method is:
[0050] (1) Take by weighing a sufficient amount of starch, lactose, microcrystalline cellulose, hydroxypropyl cellulose, calcium hydrogen phosphate and 4.8g sodium carboxymethyl starch according to the prescription and mix;
[0051] (2) measure 95% ethanol, add the sodium hydroxide of prescription quantity, stir and make dissolving; Add the povidone K30 of prescription quantity, stir and make dissolving; Ad...
Embodiment 3
[0058] Embodiment 3, a kind of Lansoprazole enteric-coated tablet, the composition of every 1000 is as follows,
[0059] Chip:
[0060]
[0061]
[0062] Isolation layer coating: hydroxypropyl methylcellulose ethanol solution (containing 1% HPMC, 3% talc, 3% PEG 6000, 1% Tween 280, 1% diethyl phthalate, 80% 95% ethanol );
[0063] Enteric layer coating: polyacrylic acid resin EudragitL100-55 aqueous suspension (containing 13% EudragitL100-55, 0.1% sodium hydroxide, 2% PEG6000, 3.4% talc, 1.5% titanium dioxide)
[0064] Above-mentioned lansoprazole enteric-coated tablet preparation method is:
[0065] (1) Take by weighing a sufficient amount of starch, lactose, microcrystalline cellulose, hydroxypropyl cellulose, calcium hydrogen phosphate and 7.38g sodium carboxymethyl starch according to the prescription and mix;
[0066] (2) measure 95% ethanol, add the sodium hydroxide of prescription quantity, stir and make dissolving; Add the povidone K30 of prescription quantity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com