Preparing method of thiocyanate functionalized ionic liquid electrolyte
A technology of ionic liquid and thiocyanate, applied in electrolytic capacitors, hybrid capacitor electrolytes, circuits, etc., can solve the problems of organic electrolytes such as flammability, low conductivity, and high viscosity, and achieve low cost, low viscosity, and equipment requirements low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The invention is a preparation method of a thiocyanate-functionalized ionic liquid electrolyte, comprising:
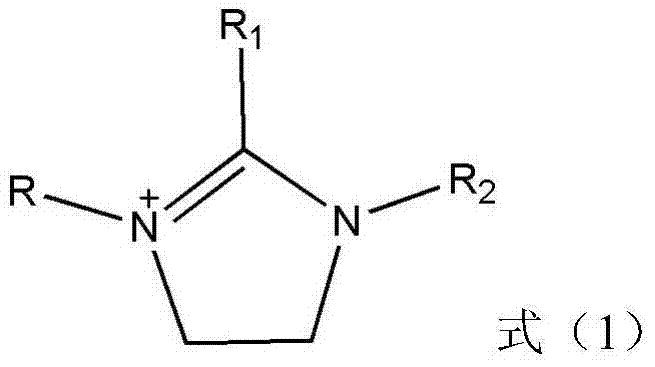
[0043] Step 1: Under the protection of nitrogen environment, take amines or phosphorus monomers and add them to the flask, add halogenated alkyl hydrocarbons to the flask dropwise, heat the mixture in the flask after the addition is completed, and raise the temperature to 60-110°C Reflux for another 4-10 hours, cool to room temperature after the reaction, wash with an organic solvent for 3 times to remove unreacted substances, remove the organic solvent by rotary evaporation, heat the product obtained above to 60-80°C and depressurize to 0.1MPa vacuum Dry for 8-12h to obtain the halide ionic liquid intermediate;
[0044] Step 2: Take the halide ionic liquid intermediate prepared above and sodium thiocyanate (NaSCN), mix according to the ratio of molar ratio 1:1-1:5, add molar amount after mixing is 2-10 times of sodium thiocyanate In the acetone solvent, stir m...
Embodiment 1
[0074] Under the protection of nitrogen environment, N-methylimidazole and n-bromoethane were added into the flask according to the molar ratio of 1:1 for mixing, and the mixture was heated to 60°C and then refluxed for 4h. After the reaction was completed, it was cooled to At room temperature, white crystals were obtained. The white crystals were washed 3 times with an organic solvent mixed with acetone and ethyl acetate in a molar ratio of 1:1-1:5 to remove unreacted substances, and then the organic solvent was removed by rotary evaporation, and the obtained The white crystals were heated to 60° C. and dried under reduced pressure to 0.1 MPa in vacuo for 10 h to obtain a white solid, the halide ionic liquid intermediate 1-ethyl-3-methylimidazolium bromide (EmimBr), with a yield of 91%.
[0075] 1-ethyl-3-methylimidazolium bromide and sodium thiocyanate (NaSCN) are mixed according to the ratio of 1:2 according to the molar ratio, after mixing, add molar weight in the acetone ...
Embodiment 2
[0077] Under the protection of nitrogen environment, N-methylimidazole and n-bromoethane were added into the flask according to the molar ratio of 1:1 for mixing, and the mixture was heated to 60°C and then refluxed for 10h. After the reaction was completed, it was cooled to At room temperature, white crystals were obtained. The white crystals were washed 3 times with an organic solvent mixed with acetone and ethyl acetate in a molar ratio of 1:1-1:5 to remove unreacted substances, and then the organic solvent was removed by rotary evaporation, and the obtained The white crystals were heated to 60° C. and dried under reduced pressure to 0.1 MPa for 10 h in vacuum to obtain a white solid, namely the halide ionic liquid intermediate 1-ethyl-3-methylimidazolium bromide (EmimBr), with a yield of 94%.
[0078] Mix 1-ethyl-3-methylimidazolium bromide and sodium thiocyanate according to the ratio of 1:2 in molar ratio, after mixing, add in acetone solvent whose molar weight is 5 time...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap