Fusion polypeptide and application of fusion polypeptide in preparing anti-tumor medicines
A technology for fusing polypeptides and drugs, applied in the field of biomedicine, can solve problems such as affecting activity, and achieve the effect of inhibiting migration and promoting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation and purification of fusion polypeptide TAT-NFAT2
[0050] (1) Determining the DYRK1A action site on NFAT2
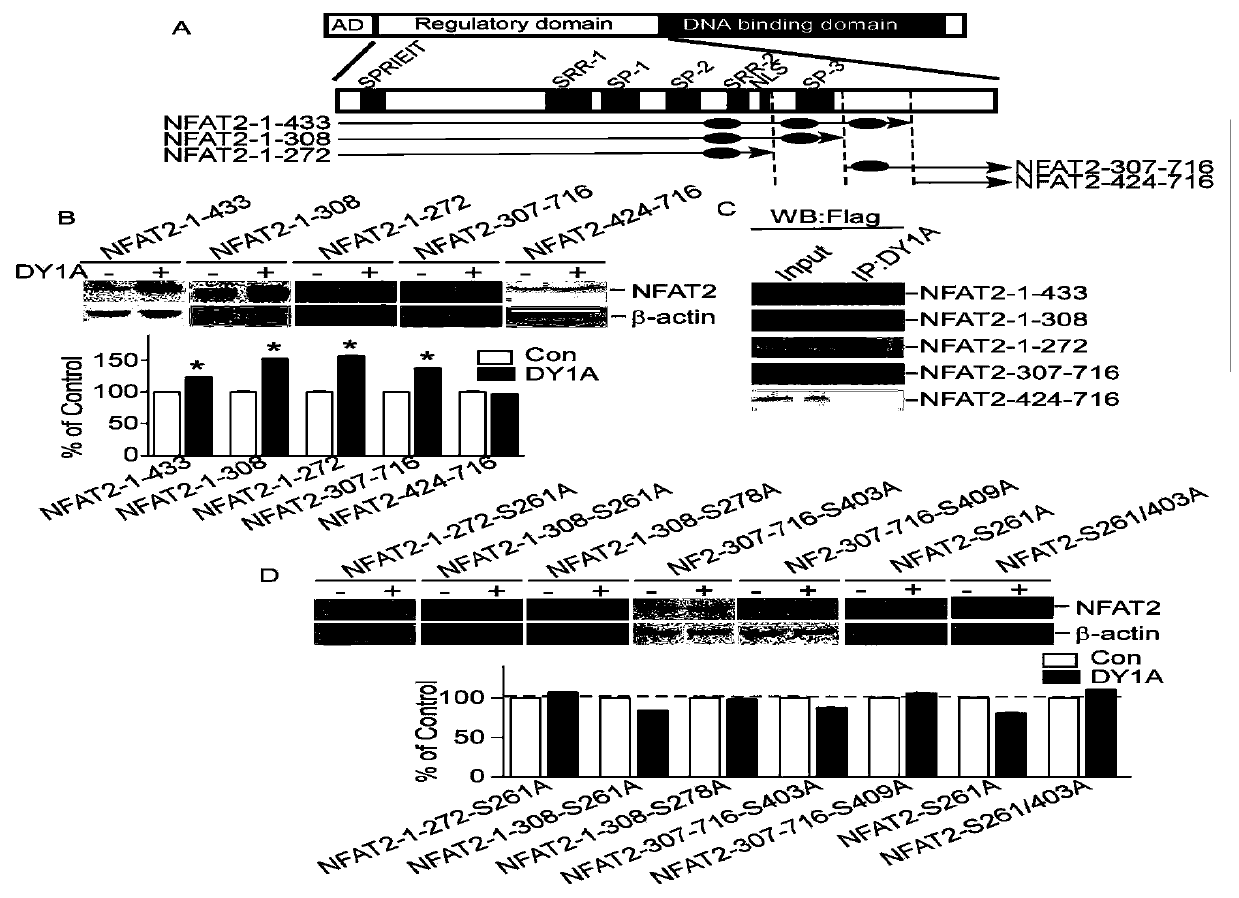
[0051] 1. The substrate of DYRK1A (the substrate refers to the protein that DYRK1A can phosphorylate, that is, the target protein) contains a conserved RPX(S / T)P domain (ie arginine-proline-any amino acid-serine or amino acid sequence of threonine-proline). Such as figure 1 As shown in A, the sequence analysis of NFAT2 protein by ClustalW2 software shows that it has three similar RPX(S / T)P domains. In order to determine the action site or target of DYRK1A on NFAT2, construct NFAT2 vector (sequence shown in SEQ ID NO.27) and NFAT2 deletion vector 1-272aa (sequence shown in SEQ ID NO.28), 1-308aa (sequence shown in SEQ ID NO.29), 1-433aa (sequence shown in SEQ ID NO.30), 307-716aa (sequence shown in SEQ ID NO.31) and 424-716aa (sequence shown in SEQ ID NO.31) and 424-716aa (sequence shown in SEQ ID NO.31) shown in NO.32).
[0052] The NF...
Embodiment 2
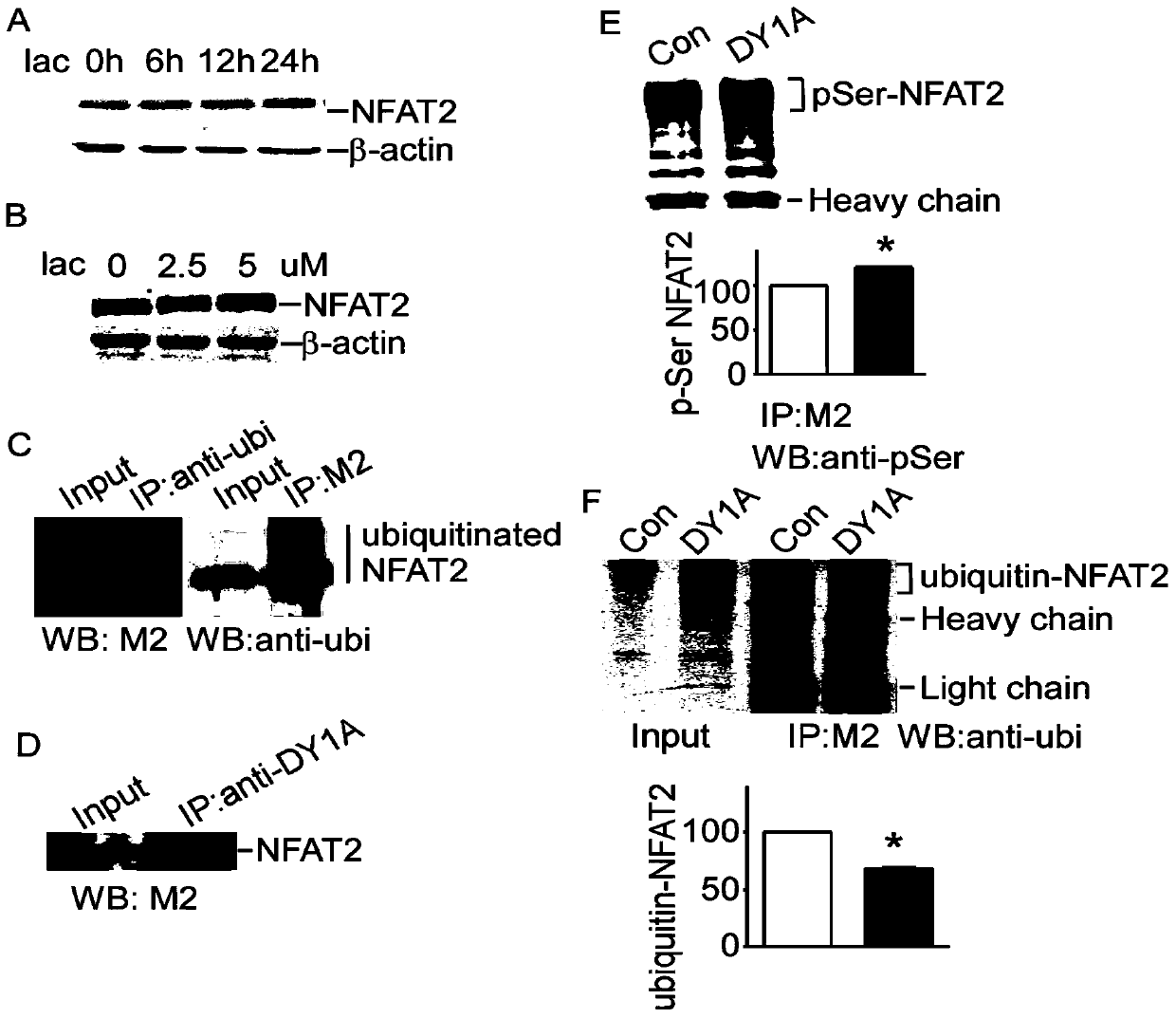
[0079] Example 2: The fusion polypeptide TAT-NFAT2 inhibits the effect of DYRK1A on NFAT2 in tumor cells
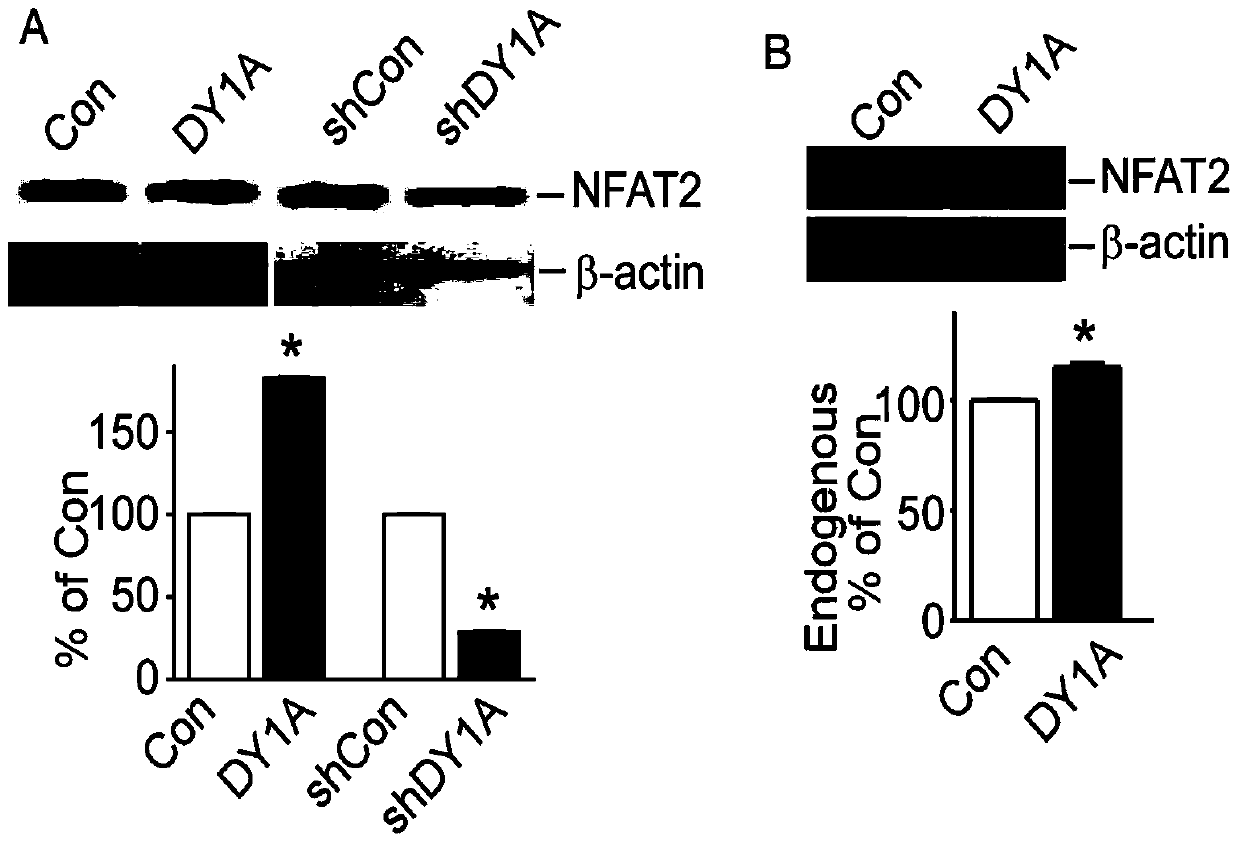
[0080] (1) DYRK1A increases the expression of NFAT2 protein in tumor cell HEK293
[0081] 1. pGFP-V-RS-shDYRK1A (it is the knockout vector of DYRK1A, which can express a 20-length nucleotide sequence, which can degrade the mRNA of DYRK1A and inhibit the protein expression of DYRK1A) was constructed by our laboratory (EST negative feedback loop regulates DYRK1A. Journal of Biochemistry (2011) 286, 10755-10763 (see literature Lu, M., Zheng, L., Han, B., Wang, L., Wang, P., Liu, H., and Sun, X. (2011) The Journal of biological chemistry 286, 10755-10763).
[0082] Using Liposome 2000 (11668-027; Invitrogen), p-NFAT2mycflag was co-transfected with pCMV-DYRK1A and pGFP-V-RS-shDYRK1A into HEK293 cells, respectively, at 37°C, 5% CO 2 Cells were harvested after 48 hours of culture in the environment, and the cells were lysed with RIPA lysate containing 0.1% SDS to obtain the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com