CaMKII, IP3R, CALCINEURIN, P38 AND MK2/3 INHIBITORS TO TREAT METABOLIC DISTURBANCES OF OBESITY
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Regulation of Hepatocyte Glucose Production by a Calcium Sensing Enzyme, CaMKII
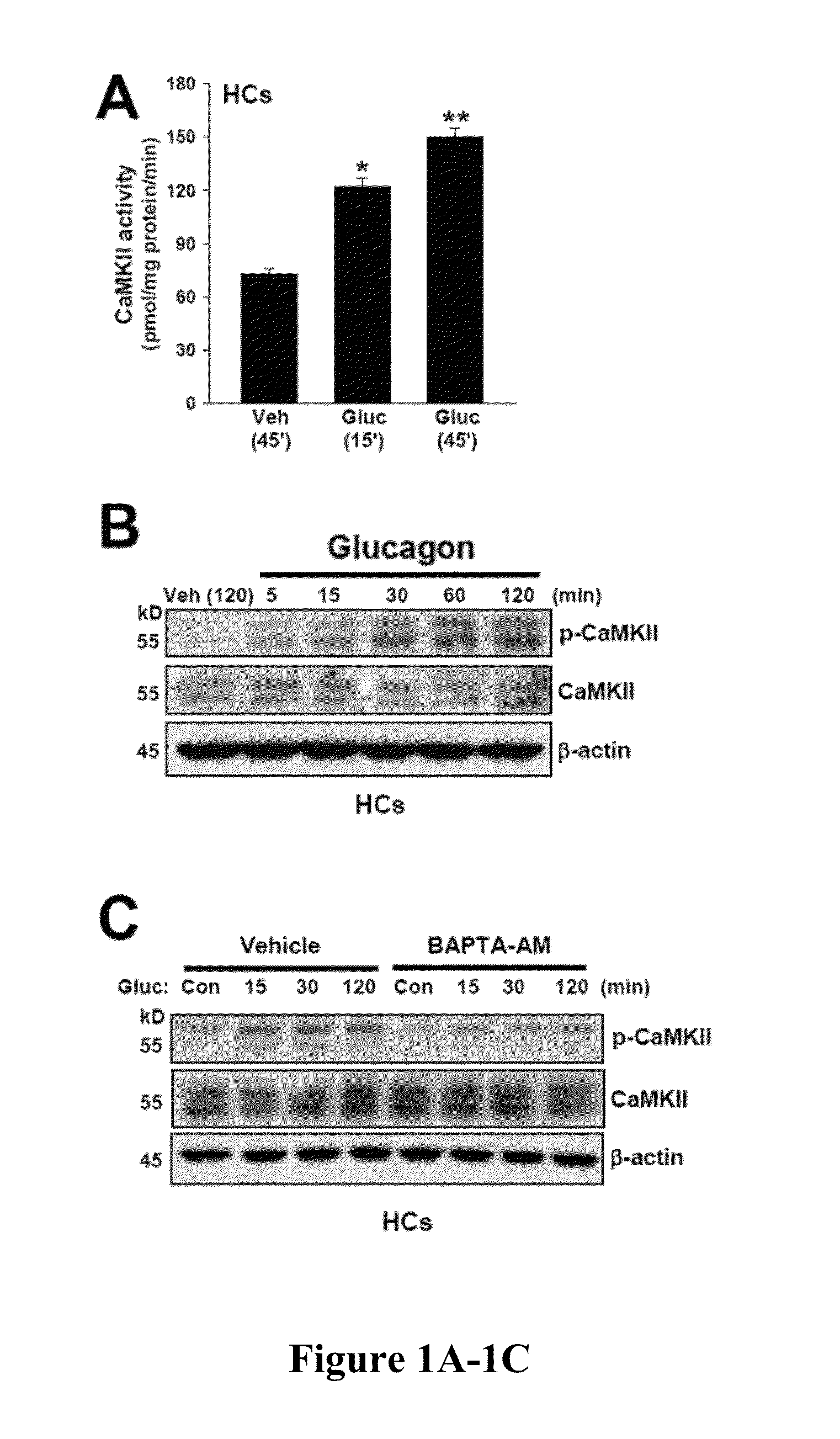
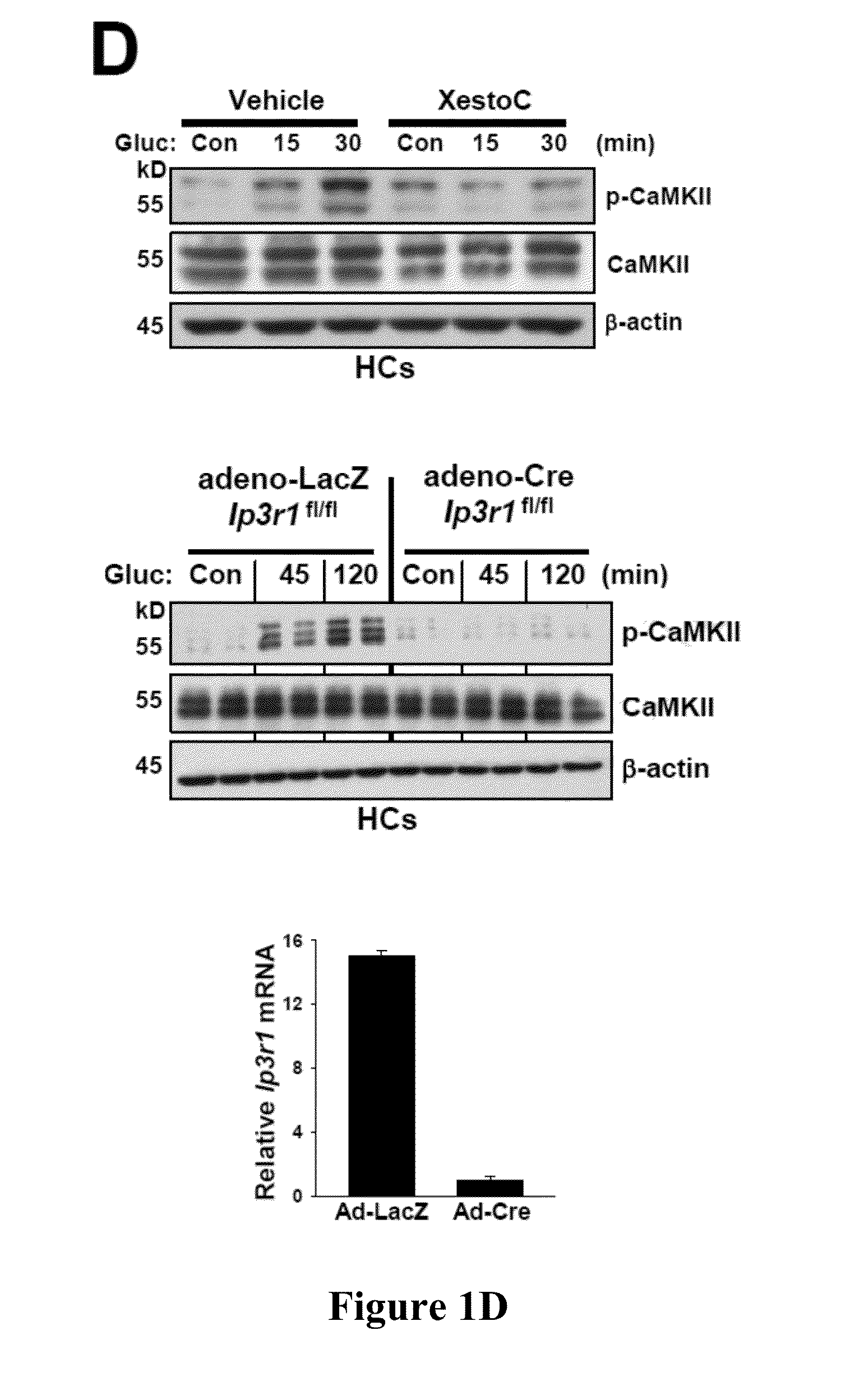
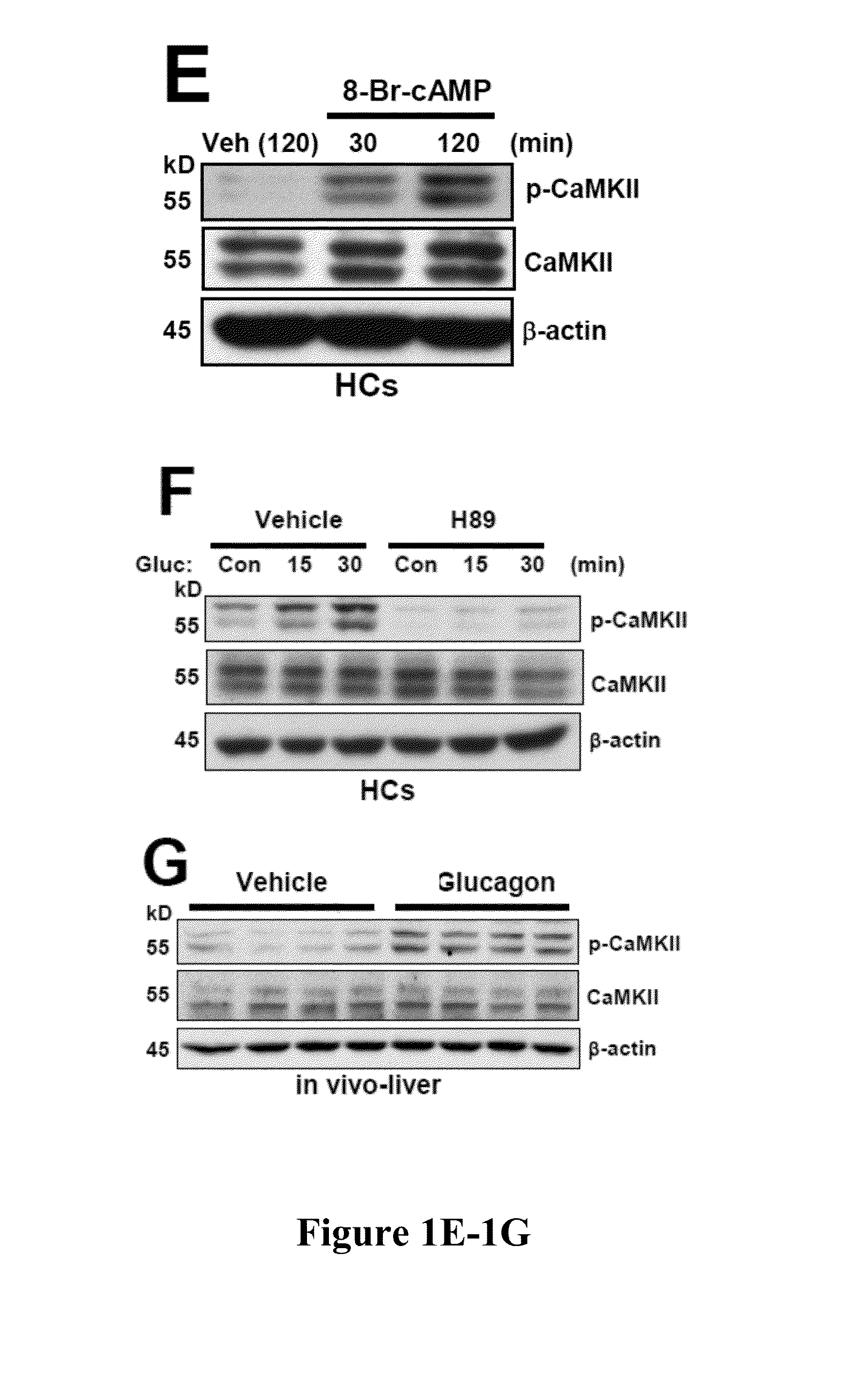
[0419]Hepatic glucose production is crucial for glucose homeostasis. Several transcription factors and co-activators have been shown to regulate this process, however, the underlying mechanisms have not been fully elucidated. As described herein, a calcium sensing enzyme, CaMKII, is activated in a calcium- and IP3R-dependent manner by cAMP and glucagon in primary hepatocytes and by glucagon and fasting in vivo. Genetic deficiency or inhibition of CaMKII blocks nuclear translocation of FoxO1, impairs fasting- and glucagon / cAMP-induced glycogenolysis and gluconeogenesis, and lowers blood glucose levels. Conversely, adenoviral expression of constitutively active CaMKII induces genes involved in gluconeogenesis and glycogenolysis, stimulates glucose production both in vitro and in vivo, and raises blood glucose levels. The suppressive effect of CaMKII deficiency on glucose metabolism is abrogated by transduct...
example 2
Regulation of Hepatocyte Glucose Production by a Calcium Sensing Enzyme, CaMKII
[0420]Hepatic glucose production is crucial for glucose homeostasis. Several transcription factors and co-activators have been shown to regulate this process, however, the underlying mechanisms have not been fully elucidated. As described herein, a calcium sensing enzyme, CaMKII, is activated in a calcium- and IP3R-dependent manner by cAMP and glucagon in primary hepatocytes and by glucagon and fasting in vivo. Genetic deficiency or inhibition of CaMKII blocks nuclear translocation of FoxO1, impairs fasting- and glucagon / cAMP-induced glycogenolysis and gluconeogenesis, and lowers blood glucose levels. Conversely, adenoviral expression of constitutively active CaMKII induces genes involved in gluconeogenesis and glycogenolysis, stimulates glucose production both in vitro and in vivo, and raises blood glucose levels. Importantly, the suppressive effect of CaMKII deficiency on glucose metabolism is abrogated...
example 3
Regulation of Hepatocyte Glucose Production by a Calcium Sensing Enzyme, CaMKII
[0421]Liver is the main organ responsible for maintaining euglycemia under conditions of nutrient deprivation. During the early stages of fasting, liver uses glycogen stores to mobilize glucose (Radziuk and Pye, 2001). As fasting progresses, de novo synthesis of glucose from non-carbohydrate precursors, gluconeogenesis, becomes the main contributor to hepatic glucose production (Klover and Mooney, 2004). Glucose production is also regulated by substrate flux through glycolysis, glycogen synthesis, and glycogenolysis. These changes occur rapidly in response to direct hormonal signaling. In addition, both insulin and glucagon affect transcription of glycogenolytic and gluconeogenic enzymes, glucose-6-phosphatase (G6pc) and phosphoenolpyruvate carboxykinase (Pck1), respectively (Pilkis and Granner, 1992). During fasting, glucagon and its downstream effector, cAMP, induce changes in the subcellular localizati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
| Fluorescence | aaaaa | aaaaa |
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com