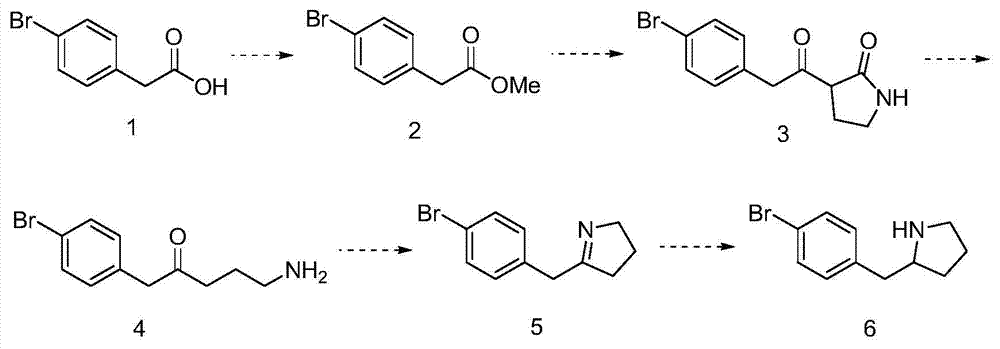
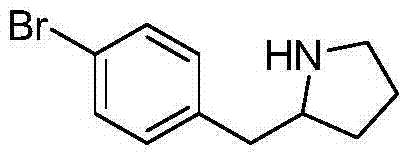
2-(4-bromine benzyl) pyrrolidine preparation method
A technology of pyrrolidine and bromobenzyl is applied in the field of novel preparation of pharmaceutical intermediates and can solve problems such as difficulty in synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
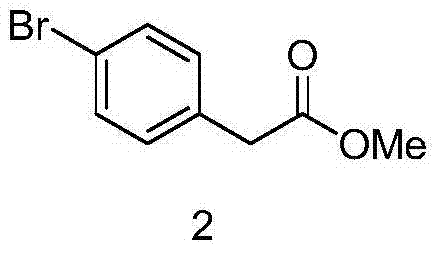
[0024] (1) Synthesis of 2-(4-bromophenyl)methyl acetate
[0025] Add 20g of p-bromophenylacetic acid to 400ml of methanol, add 2g of toluenesulfonic acid, stir at room temperature for 24 hours, concentrate, add water and ethyl acetate to extract, separate, dry, concentrate, and separate the residue on a column to obtain 22g of 2 -methyl (4-bromophenyl)acetate.
[0026] (2) Synthesis of 3-(2-(4-bromophenyl)acetyl)pyrrolidin-2-one
[0027] Add 20g of methyl 2-(4-bromophenyl)acetate to 320ml of o-xylene, then add 14g of 2-ethylbutamidine, add 9g of potassium carbonate, heat to reflux overnight, cool to room temperature, concentrate, then add water and dichloromethane, extracted and separated, collected the organic phase, separated, dried and concentrated, and the residue was separated on a silica gel column to obtain 15 g of 3-(2-(4-bromophenyl)acetyl)pyrrolidin-2-one.
[0028] (3) Synthesis of 5-amino-1-(4-bromophenyl)pentan-2-one
[0029] Put 14g of 3-(2-(4-bromophenyl)acety...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com