Solid forms of an epidermal growth factor receptor kinase inhibitor
A form of solid technology, used in plant growth regulators, biocides, animal repellants, etc., to solve problems such as dose-limiting toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0227] Preparation Form A
[0228] Approximately 120 mg of Compound 1 was weighed into a vial and slurried in approximately 2 ml of acetonitrile. This slurry was temperature cycled between about 0°C and ambient (about 22°C) in a 2 hour cycle with stirring for a period of 2-3 days. Keep samples at about 2-5°C overnight. The solid material was separated and allowed to dry under vacuum for 7 days.
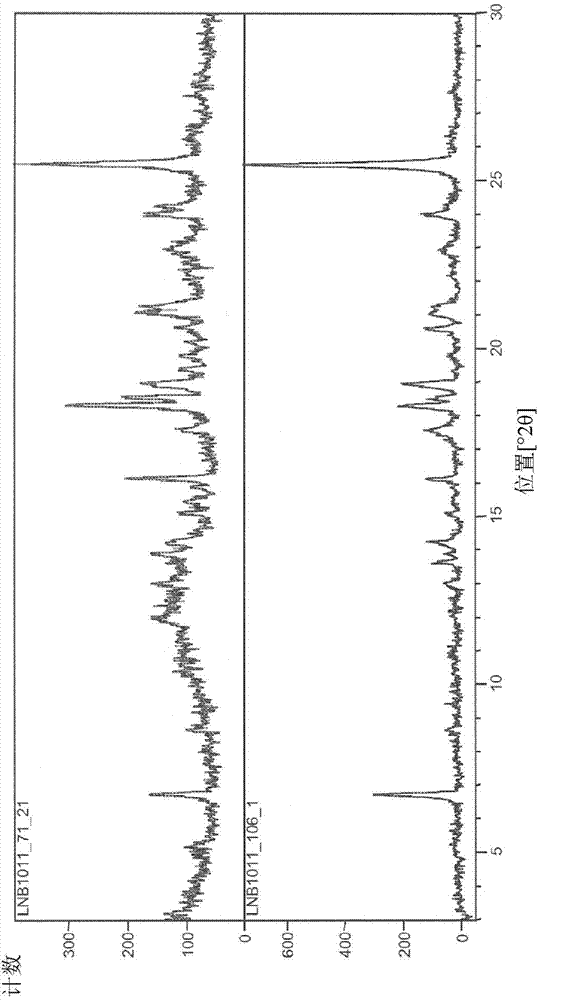
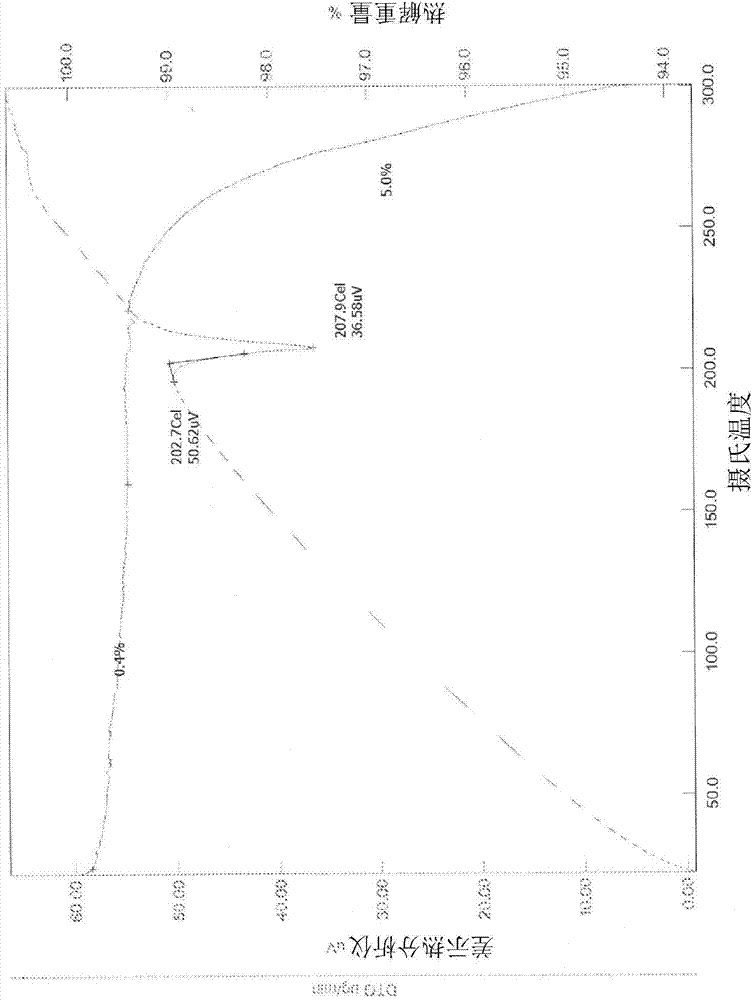
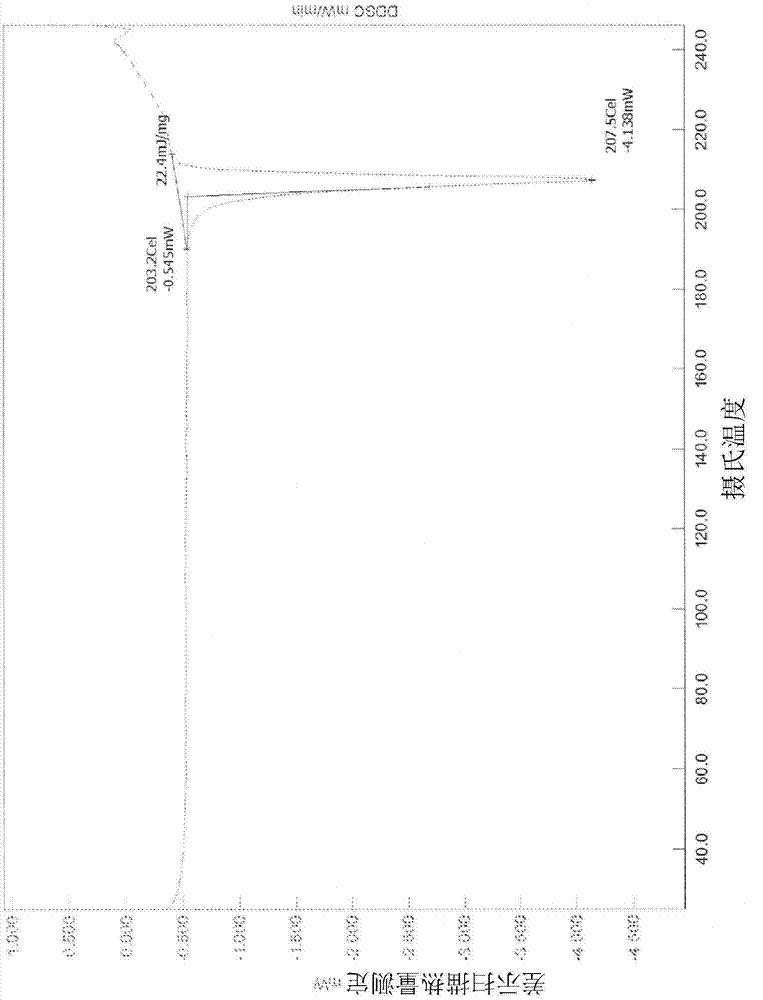
[0229] XRPD analysis ( figure 1 ) shows that the material is crystalline. PLM analysis (not shown) indicated very fine birefringent needles. TGA / DTA ( figure 2 ) shows a 0.4% weight loss from start to about 150°C, probably due to unbound solvent. No significant weight loss was seen prior to degradation. DSC analysis ( image 3 ) shows a single endotherm at onset about 203.2°C (peak 207.5°C) due to melting. IR analysis ( Figure 4 ) is consistent with the input free base substance. in deuterated DMSO 1 H NMR (not shown) showed a spectrum consistent with the input free bas...
example 2
[0232] Preparation Form B
[0233] About 120 mg of compound 1 was weighed into a vial and slurried in about 2 ml of tetrahydrofuran. This slurry was temperature cycled between about 0°C and ambient (about 22°C) in a 2 hour cycle with stirring for a period of 2-3 days. Keep samples at about 2-5°C overnight. The solid material was separated and allowed to dry under vacuum at ambient for 7 days and at 40° C. for 2 days.
[0234] XRPD analysis ( Figure 6 ) shows that the resulting material is crystalline. PLM analysis (not shown) indicated birefringent rod crystals. TGA / DTA ( Figure 7 ) shows no significant weight loss before degradation after 7 days of ambient drying under vacuum and a further 2 days at 40°C. DSC analysis ( Figure 8 ) shows an endotherm at onset 153.6°C (peak 157.6°), followed directly by an exotherm at peak 161.3°C, indicating a polymorphic transition. There was another small endotherm at peak 186.0°C, followed by a final endotherm at onset 203.9°C (p...
example 3
[0238] Preparation Form C
[0239] Approximately 120 mg of Compound 1 was weighed into a vial and slurried in approximately 100 μl DMF. The slurry was temperature cycled between about 0°C and ambient (about 22°C) in a 2 hour cycle with agitation. After about 2 hours, another 300 μl of DMF was added. The temperature cycle was continued for a period of 2-3 days. Keep samples at about 2-5°C overnight. The solid material was separated and allowed to dry under vacuum at ambient for 7 days and at 40° C. for 2 days.
[0240] XRPD analysis ( Figure 12 ) shows that the material is crystalline. PLM analysis (not shown) indicated birefringent rod crystals. TGA / DTA ( Figure 13 ) showed a weight loss of about 8.2% after 7 days of ambient drying under vacuum and a further 2 days at 40°C (the monosolvate required 11.6 wt% DMF). DSC analysis ( Figure 14 ) showed a broad endotherm between 85-125 °C, consistent with weight loss in TGA. A final endotherm was seen with onset at about...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com