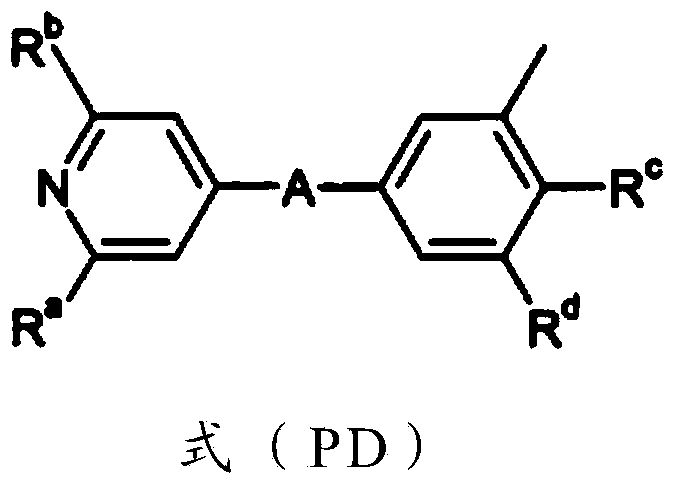
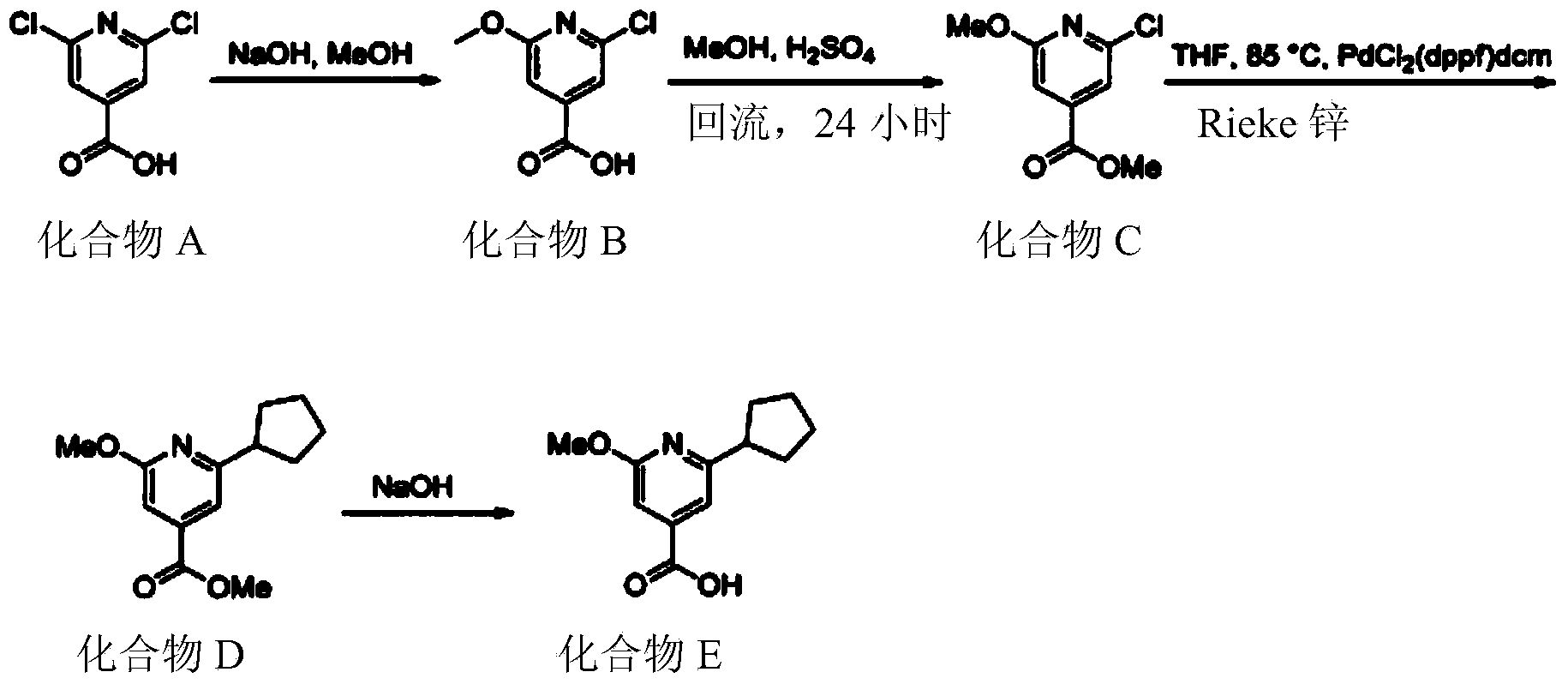
New process for the preparation of 2-cyclopentyl-6-methoxy-isonicotinic acid
A technology of cyclopentanecarboxylic acid and cyclopentyl, which is applied in the field of preparation of 2-cyclopentyl-6-methoxy-isonicotinic acid, can solve problems such as expensive, lack of manufacturing, and insufficient efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0195] The following examples illustrate the invention without limiting its scope.
[0196] All temperatures are external and are in °C. compound is used 1 H-NMR (400MHz) or 13 C-NMR (100 MHz) analytical features (Bruker; chemical shifts are given in ppm relative to the solvent used; multimodality: s=singlet, d=doublet, t=triplet, p=pentamodal, hex=six Peak, hept=seven peaks, m=multiple peaks, br=broad peak, coupling constant is in Hz), the internal standard substance of quantitative NMR is 1,4-dimethoxybenzene; adopt LC-MS (plus Install Agilent 1200 binary pump and Agilent MS detector G1956B of DAD), t R is in minutes, or using GC-MS (Thermo Scientific, Trace Ultra, DSQ II detector), t R is in minutes.
[0197] GC-MS method:
[0198]
[0199] LC-MS method:
[0200]
[0201] Abbreviations (as used in this article):
[0202] aq. aqueous solution
[0203] b.p. boiling point
[0204] Burgess Reagent Methoxycarbonylsulfamoyltriethylammonium hydroxide
[0205] DCM ...
example 2
[0262] 2-cyclopentyl-6-hydroxyisonicotinic acid
[0263]
[0264]Potassium tert-butoxide (220 g, 1.1 equiv) and THF (3 L) were charged to a 10 L reactor. The solution was cooled to -20°C. A mixture of diethyl oxalate (260 g, 1 eq) and 1-cyclopentylethanone (200 g, 1.78 mol, 1 eq) was added at a temperature below -18°C. The reaction mixture was stirred at -10°C for 30 minutes and then heated to 15°C. Cyanoacetamide (180 g, 1.2 equiv) was added to the mixture. The mixture was stirred at 22°C for 20 hours. Water (600ml) was added and the reaction mixture was concentrated on a rotary evaporator at 60°C under reduced pressure. 3.4 L of solvent was removed. 32% HCl (5 L) was added to the reactor and heated to 50°C. The residue was added to HCl solution at a temperature between 44 and 70°C. The mixture was heated to 100°C for 22 hours. 2.7 L of solvent were removed at an external temperature of 135°C and a pressure of about 400 mbar. Water (2.5 L) was added to dilute the ...
example 3
[0266] Methyl 2-cyclopentyl-6-hydroxyisonicotinate
[0267]
[0268] 2-cyclopentyl-6-hydroxyisonicotinic acid (1520.5g, 7.3mol, 1 equivalent), methanol (15.2L), trimethyl orthoformate (1.56L, 2 equivalents) and sulfuric acid (471ml, 1.2 equivalents) Mix and heat to reflux at 20°C for 18 hours. 10 L of solvent was removed at an external temperature of 95°C and a pressure of about 800 mbar.
[0269] The mixture was cooled to 20°C and added to water (7.6 L) at 50°C. The suspension was diluted by water (3.8 L), cooled to 10 °C and filtered. The filter cake was washed by water (3.8 L). The product was dried to obtain a light yellow solid; Yield: 1568 g (97%); Purity (LC-MS): 100% a / a; LC-MS: t R = 1.158 minutes, [M+1] + = 222; 1 H NMR (deuterated DMSO)δ=11.98(br,1H),6.63(m,1H),6.39(s,1H),3.83(s,3H),2.91(m,1H),1.99(m,2H) ,1.72(m,2H),1.58(m,4H).
[0270] Example 4a
[0271] 2-Chloro-6-cyclopentylisonicotinic acid methyl ester
[0272]
[0273] 2-Cyclopentyl-6-hydroxyi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com