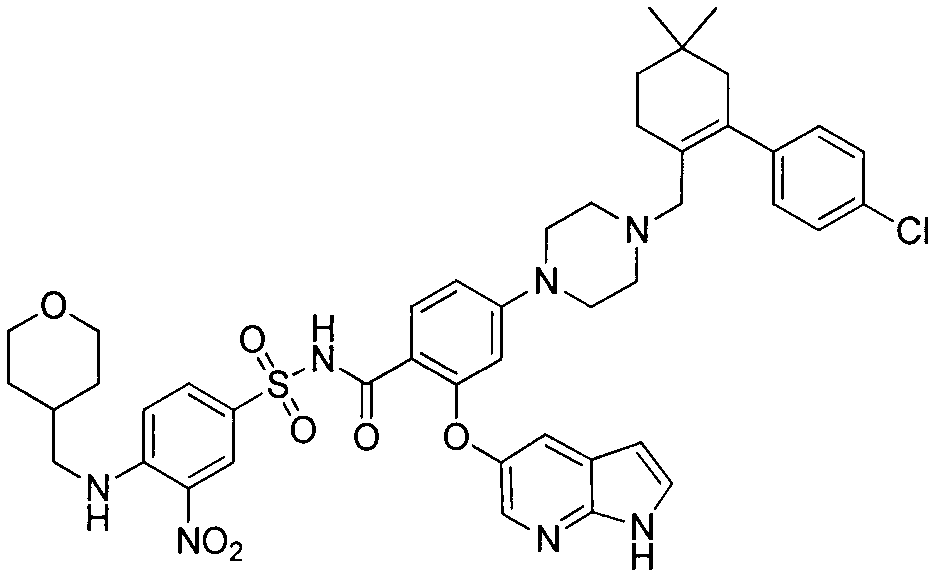
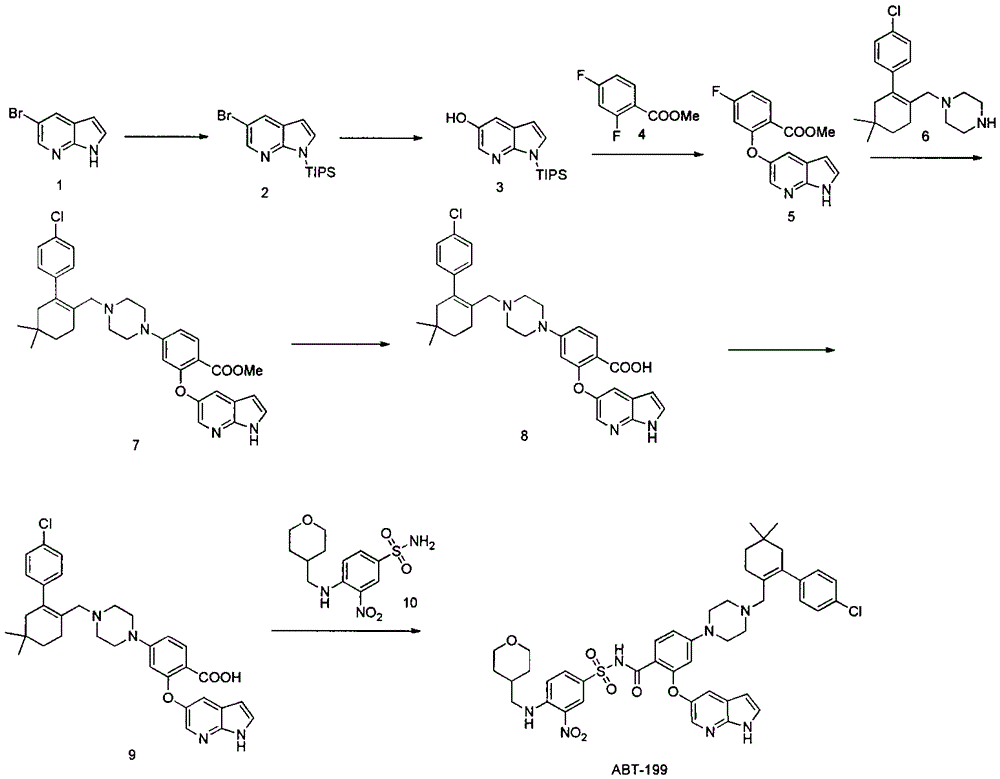
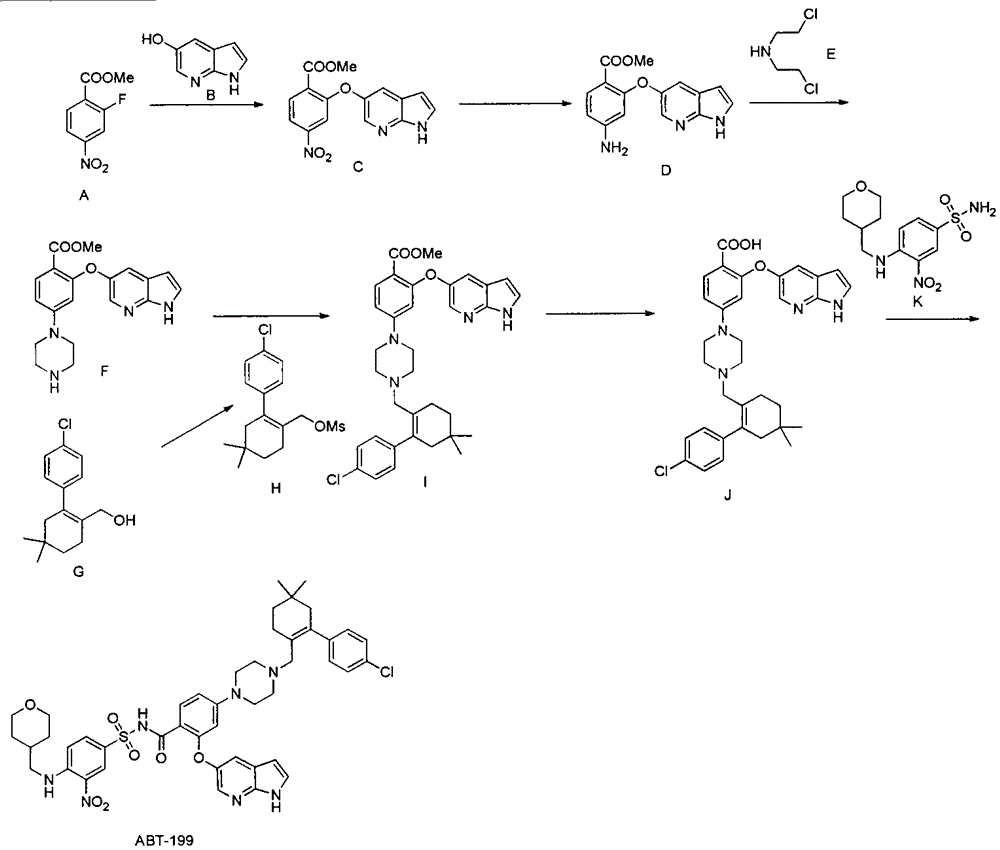
Synthesis of bcl-2 inhibitor abt-199
A technology of ABT-199 and bcl-2, applied in the field of drug synthesis, can solve the problems of inability to purify, low yield, low yield of compound 5, etc., and achieve the effects of simple steps and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation of embodiment one compound (F)
[0020]
[0021] Step 1: Synthesis of compound (C)
[0022] Add 50.0g of methyl 2-fluoro-4-nitrobenzoate into a 1L three-necked flask, dissolve it in 250ml of N'N-dimethylformamide, add 33.6g of 5-hydroxy-7-azaindole in sequence, Potassium carbonate was 34.7g, and the reaction liquid was heated at 50°C for 2 hours under the protection of nitrogen gas, then poured into 2L of ice water, extracted three times with ethyl acetate, and the organic phase was dried with saturated sodium chloride and then spin-dried to obtain the crude product of compound (C) 82.0 g, the crude product was directly put into the next reaction without purification.
[0023] The second step: synthetic compound (D)
[0024] Dissolve the crude product of the compound (C) in the previous step with 400ml of methanol, add 4.0g of 10% palladium carbon, and react with hydrogen gas under normal pressure. Purification directly into the next reaction.
[...
Embodiment 2
[0027] The preparation of embodiment two compound (H)
[0028]
[0029] Take 5.0 g of compound (G) (prepared according to the method in WO2012058392), dissolve it in 50 ml of dichloromethane, add 5.6 ml of triethylamine, stir and cool the reaction solution to 0-5 degrees, add 2.7 g of methanesulfonyl chloride dropwise, and complete the addition The reaction solution was raised to room temperature and reacted overnight. After the reaction was detected by TLC, water was added to quench the reaction. The organic phase was dried over anhydrous sodium sulfate and the solvent was spun off. Purification by silica gel column chromatography gave 6.5 g of compound (H), with a yield of 99%.
Embodiment 3
[0030] The preparation of embodiment three ABT-199
[0031]
[0032] The first step: synthesis of compound (I)
[0033] Add 2.5g of compound (F), 2.3g of compound (H), 1.9g of potassium carbonate, and 15ml of N'N-dimethylformamide in a 100ml three-necked flask, and react at 50 degrees under nitrogen protection. After the reaction is detected by TLC The reaction solution was poured into ice water, extracted twice by adding ethyl acetate, the ethyl acetate phase was dried over anhydrous sodium sulfate and then spin-dried, purified by silica gel column chromatography to obtain 3.6 g of compound (I), with a yield of 88%.
[0034] The second step: synthesis of compound (J)
[0035] Add 1.0 g of compound (I) to a 10 ml single-necked bottle, followed by adding 5 ml of water, 5 ml of ethanol, 5 ml of tetrahydrofuran, and 136 mg of sodium hydroxide. pH4-5, extracted three times with ethyl acetate, dried over anhydrous sodium sulfate and then spin-dried to obtain 907 mg of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com