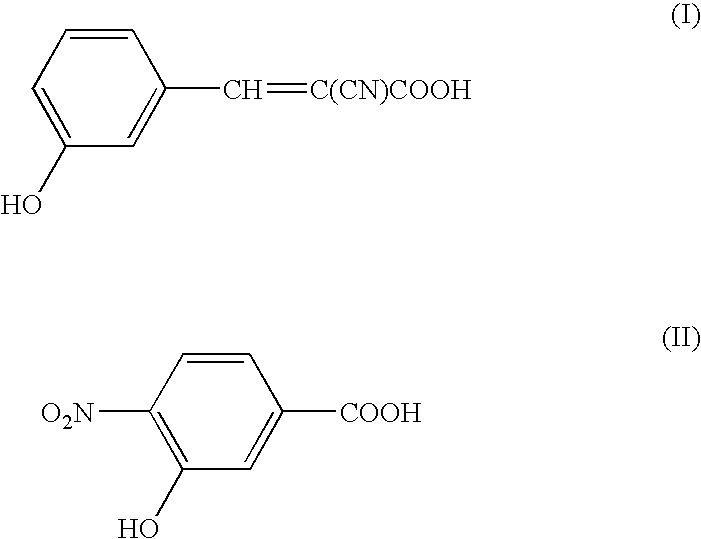
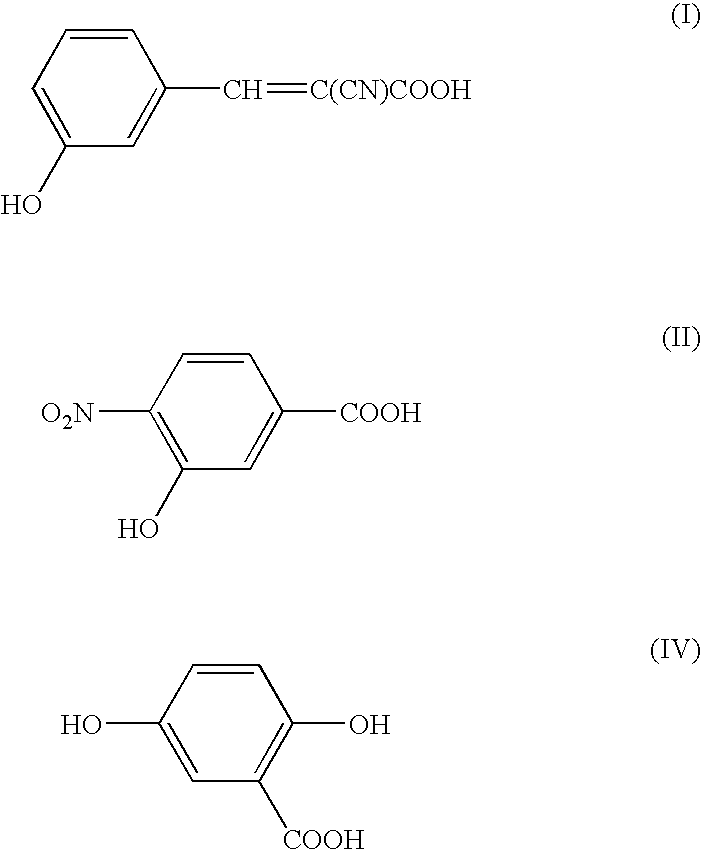
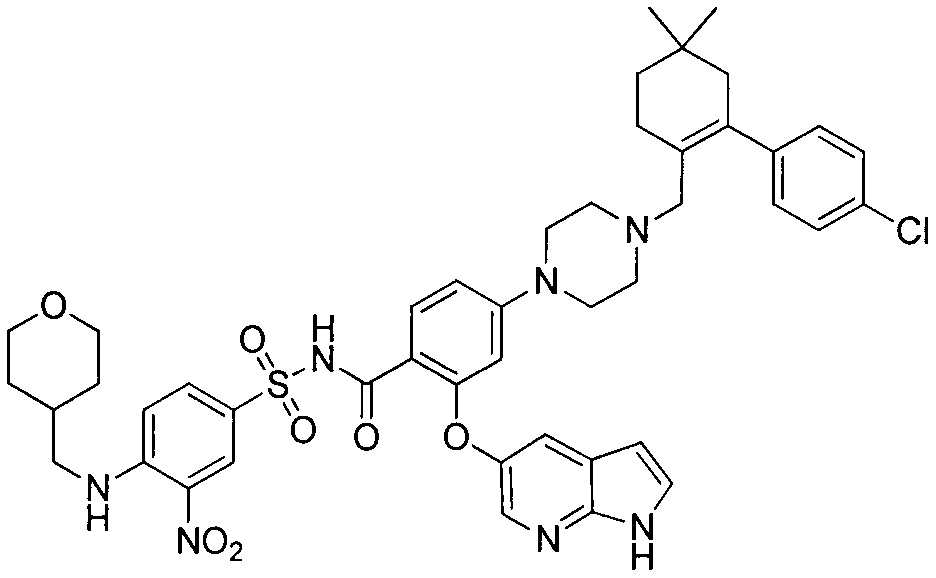
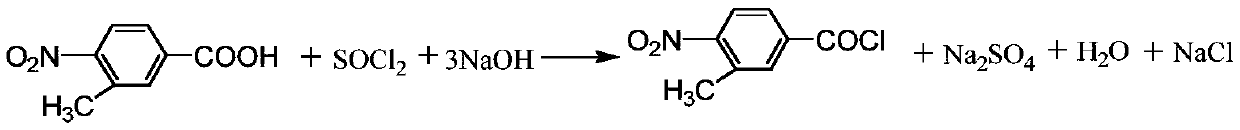
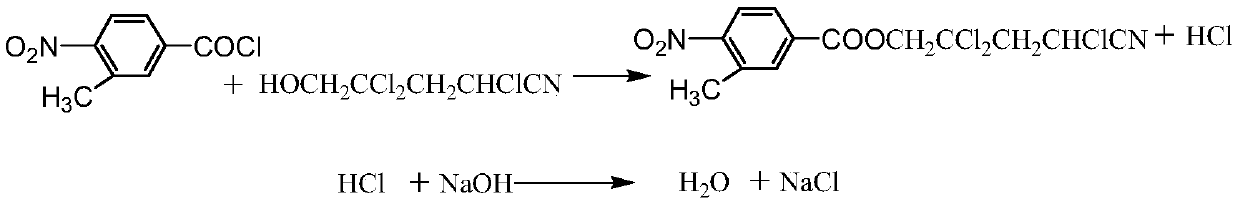
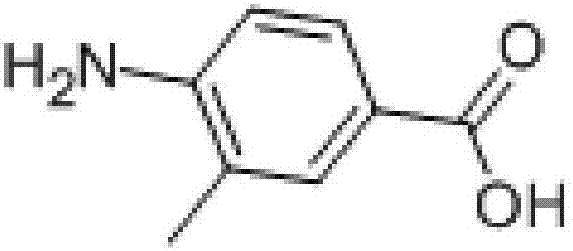
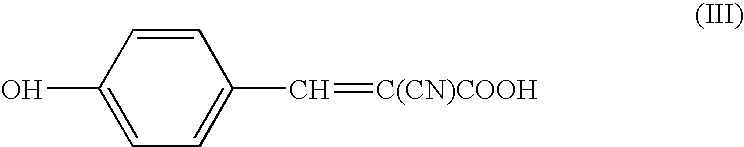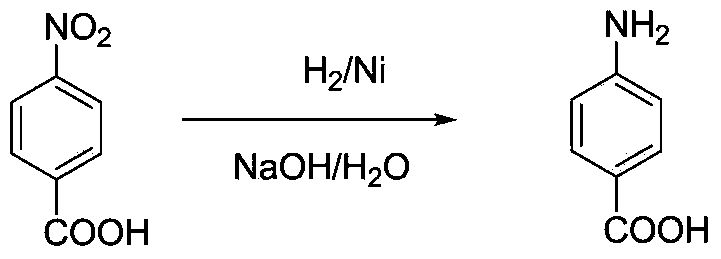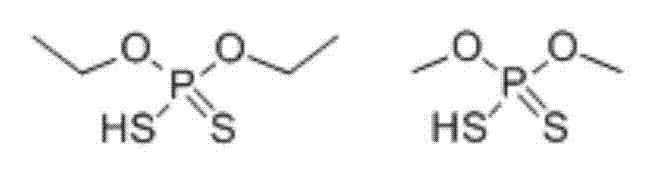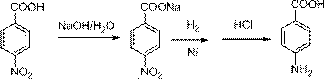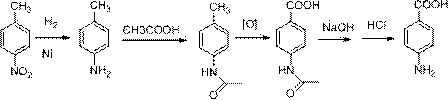Patents
Literature
54 results about "4-Nitrobenzoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
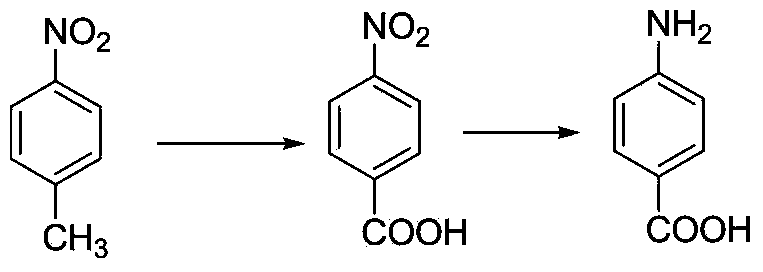
4-Nitrobenzoic acid is an organic compound with the formula C₆H₄(NO₂)CO₂H. It is a pale yellow solid. It is a precursor to 4-nitrobenzoyl chloride, the precursor to the anesthetic procaine and folic acid. It is also a precursor to 4-aminobenzoic acid.
Method for measuring hydrophobic peptides using maldi mass spectrometer
InactiveUS20050224710A1Efficient ionizationIon sources/gunsIsotope separationMass spectrometric2-nitrobenzenesulfenyl chloride
The present invention provides a method capable of efficiently ionizing hydrophobic peptides in MALDI-IT, MALDI-IT-TOF, and MALDI-FTICR mass spectrometers. A method of measuring a peptide with a mass spectrometer having a MALDI (Matrix Assisted Laser Desorption / Ionization) ion source, using α-cyano-3-hydroxycinnamic acid or 3-hydroxy-4-nitrobenzoic acid as a matrix. Preferably, a peptide derivatized with 2-nitrobenzenesulfenyl chloride is measured with a MALDI-IT, MALDI-IT-TOF, or MALDI-FTICR mass spectrometer. When 3-hydroxy-4-nitrobenzoic acid is used as a matrix, the matrix is preferably used as a mixed matrix in which α-cyano-4-hydroxycinnamic acid is combined.
Owner:SHIMADZU CORP
Synthesis of Bcl-2 inhibitor ABT-199
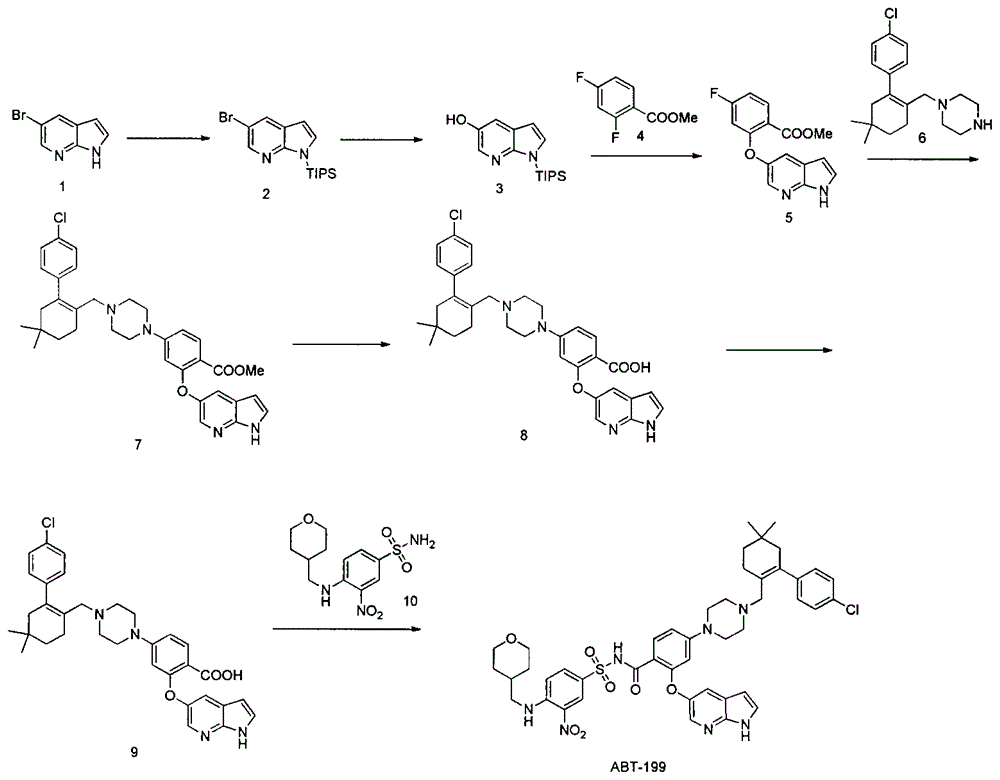
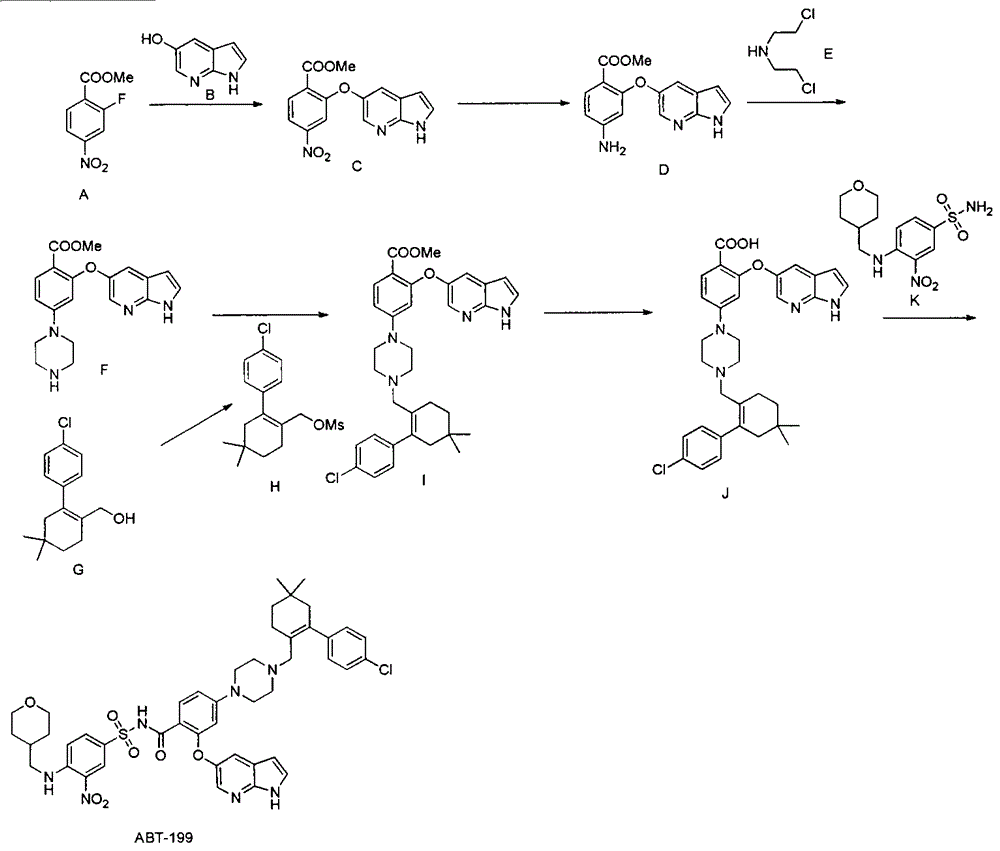
The invention discloses a synthesis method of a Bcl-2 inhibitor ABT-199. The method comprises the following steps: by using methyl 2-fluoro-4-nitrobenzoate and 5-hydroxy-7-azaindole as raw materials, carrying out substitution, reduction, cyclization, substitution, hydrolysis, condensation and the like to synthesize the ABT-199. The method has the characteristics of mild reaction conditions, simple operating technique, low cost and high yield.
Owner:南京正济医药销售有限公司
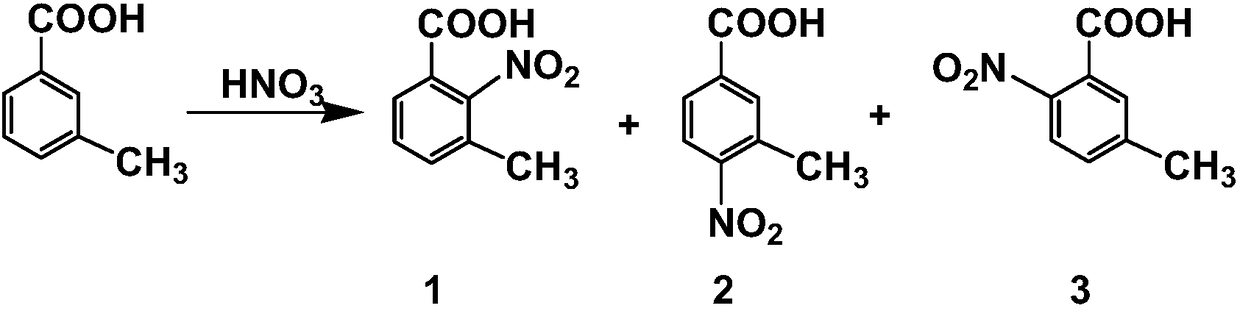
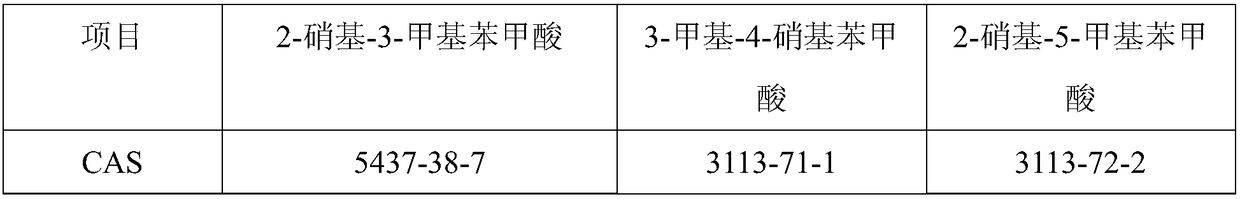
Preparation method of highly selective 3-methyl-2-nitrobenzoic acid
InactiveCN106496038AEasy to cleanReduce pollutionOrganic compound preparationCarboxylic acid esters preparationNitrationMedicinal chemistry
The invention discloses a preparation method of 3-methyl-2-nitrobenzoic acid. 3-methylbenzoic acid alkyl ester is used as raw material in nitrification, two-grade selectivity and high yield are realized, and the amount of waste acid was reduced. In nitrification product, only 3-methyl 2-nitrobenzoic acid alkyl ester and 3-methyl-4-nitrobenzoic acid alkyl ester are hydrolyzed after separation to obtain 3-methyl 2-Nitrobenzoic acid and 3-methyl-4-nitrobenzoic acid. The process is simple, and suitable for industrial production.
Owner:安徽安生生物化工科技有限责任公司
Comprehensive utilization method of m-toluic acid nitration solid waste
ActiveCN108218710ASimple methodEasy to operateOrganic chemistryOrganic compound preparationAlkaline waterNitration
The invention discloses a comprehensive utilization method of m-toluic acid nitration solid waste. The method comprises the following steps: (1) dissolving the m-toluic acid nitration solid waste by alkaline water so as to be clarified, and controlling the pH value of the solution to be 7.5-13 to obtain a clarified solution; (2) adjusting the pH value of the clarified solution to be 4.0-5.5 with acid to obtain slurry, performing filtration to obtain a first filtrate and a first filter cake, and pulping and filtering the first filter cake with water to obtain a 3-methyl-4-nitrobenzoic acid product; (3) adjusting the pH value of the first filtrate to be 2.5-4.0 with acid, performing filtration to obtain a second filtrate and a second filter cake, and pulping and filtering the second filter cake with water to obtain a 2-nitro-3-toluic acid product; and (4) adjusting the pH value of the second filtrate to be 0-2.5 with acid, performing filtration to obtain a third filtrate and a third filter cake, and pulping and filtering the third filter cake with water to obtain a 2-nitro-5-toluic acid product. The method disclosed by the invention is simple, easy to operate and low in cost and especially has important significance of changing wastes into valuables and facilitating environmental protection.
Owner:山东友道化学有限公司
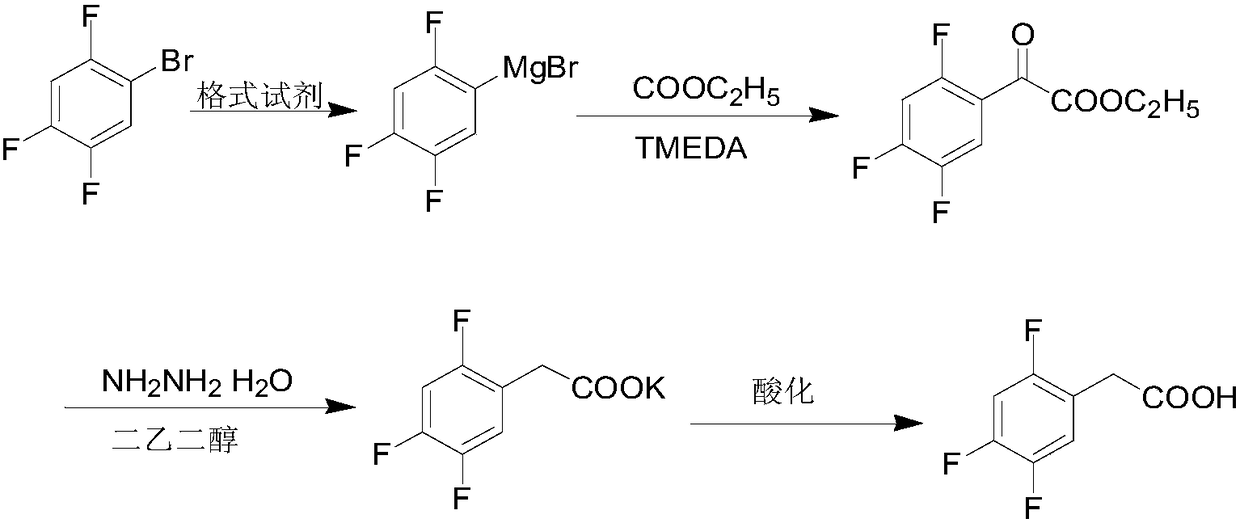
Preparation method of 2,4,5-trifluorophenylacetic acid
InactiveCN108383718AImprove conversion rateAvoid replacingOrganic compound preparationCarboxylic compound preparationStrong acidsHydrolysis
The invention relates to a preparation method of 2,4,5-trifluorophenylacetic acid, belongs to the technical field of drug intermediate synthesizing, and aims to solve the problem that existing raw materials are high in risks. The method includes: allowing 2-methyl-4-nitrobenzoic acid to have reaction with nitrite under the effect of strong acid to generate diazotizing salt, adding hydrofluoric acid or fluoborate to perform fluorination, and performing pyrolysis to obtain a compound as shown in formula II; in the presence of an acid-binding agent, allowing the compound as shown in formula II tohave acylation reaction with halogenated acetyl chloride to obtain a compound as shown in formula III; under the effect of alkali metal alkoxide, allowing the compound as shown in formula III to havereaction with alcohol to obtain a compound as shown in formula IV; performing hydrolysis and decarboxylation on the compound as shown in formula IV in an acid system, performing reduction, and then performing diazotization and pyrolysis to obtain the 2,4,5-trifluorophenylacetic acid. The preparation method has the advantages that raw material conversion rate is increased, quality requirements onproduct yield and purity are satisfied, and high final-product total yield is achieved.
Owner:江苏八巨药业有限公司
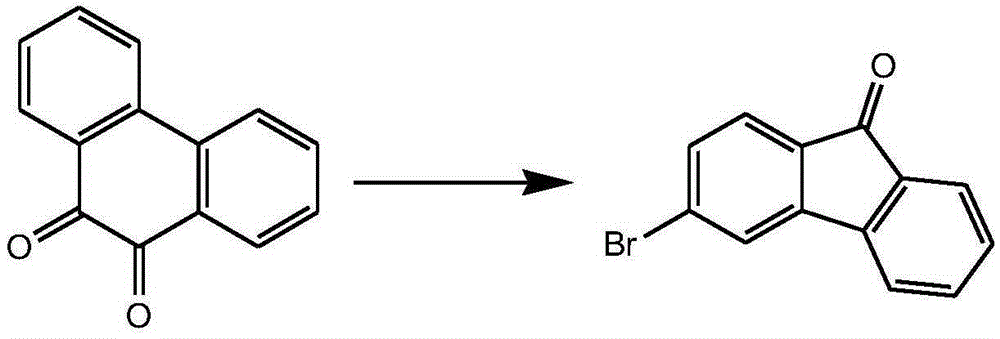
Preparation method of 3-halogenated fluorenone compound
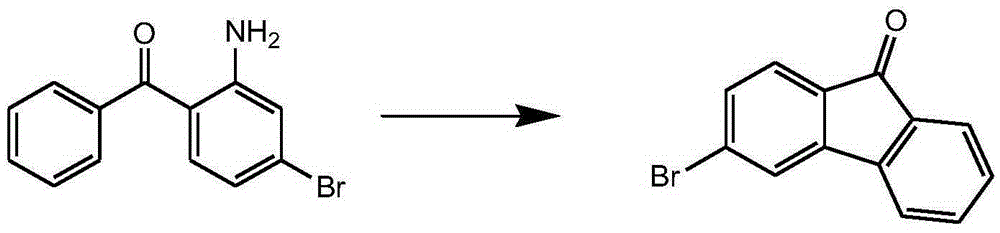
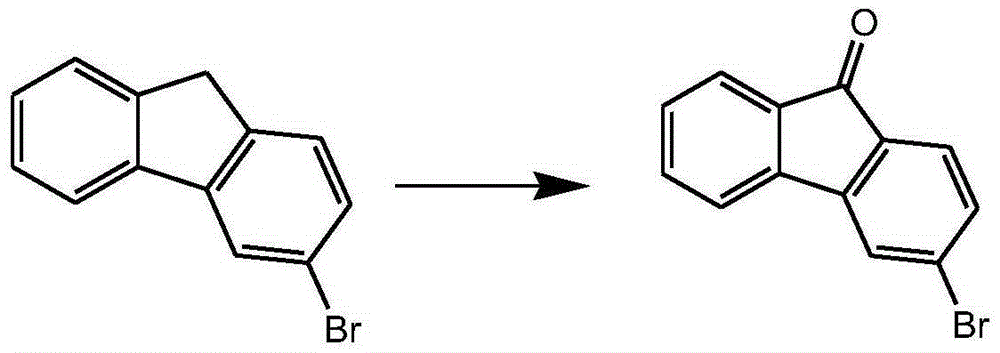
InactiveCN105294415AMild reaction conditionsEasy to operateOrganic compound preparationCarboxylic acid esters preparationBromineSuzuki reaction
The invention relates to a preparation method of a 3-halogenated fluorenone compound. According to the method, 2-bromine-4-nitrobenzoic acid methyl ester is used as a starting material, and through Suzuki reaction, reduction reaction, diazotization reaction and ring closing reaction, the 3-halogenated fluorenone compound is prepared. When the method is adopted for synthesizing the 3-halogenated fluorenone, the cost is low; the condition is mild; the operation is simple; the preparation method is suitable for laboratories and industrial large-scale preparation.
Owner:烟台九目化学股份有限公司
Preparation method of 3-chloro(bromo)-6-nitroisoquinoline
The invention relates to a preparation method of 3-chloro(bromo)-6-nitroisoquinoline. The preparation method comprises that 2-chloro-4-nitrobenzoic acid as a raw material undergoes a nucleophilic substitution reaction to produce 2-(2-methoxy-2-oxoethyl)-4-nitrobenzoic acid, the 2-(2-methoxy-2-oxoethyl)-4-nitrobenzoic acid is hydrolyzed to form 2-(carboxymethyl)-4-nitrobenzoic acid, the 2-(carboxymethyl)-4-nitrobenzoic acid, acetyl chloride and ammonium hydroxide undergo a reaction to produce 2-(2-amino-2-oxoethyl)-4-nitrobenzoic acid, the 2-(2-amino-2-oxoethyl)-4-nitrobenzoic acid undergoes a cyclization reaction to produce 6-nitroisoquinolin-1,3(2H,4H)-dione, the 6-nitroisoquinolin-1,3(2H,4H)-dione undergoes a chlorination (bromination) reaction to produce 1,3-dichloro(bromo)-6-nitroisoquinoline, because of activity of isoquinoline at the first site, chlorine(bromine) at the first site is replaced by methoxybenzylamine so that 3-chloro(bromo)-N-(4-methoxybenzyl)-6-nitroisoquinolin-1-amine is formed, the p-methoxybenzyl group is removed by trifluoroacetic acid so that 3-chloro(bromo)-6-nitroisoquinolin-1-amine is formed, 1-amine is subjected to diazotization, and the hydrogen produced by diazotization is removed by heating so that a product is obtained. The preparation method is convenient for operation and has a high yield in each process.
Owner:烟台宁远药业有限公司
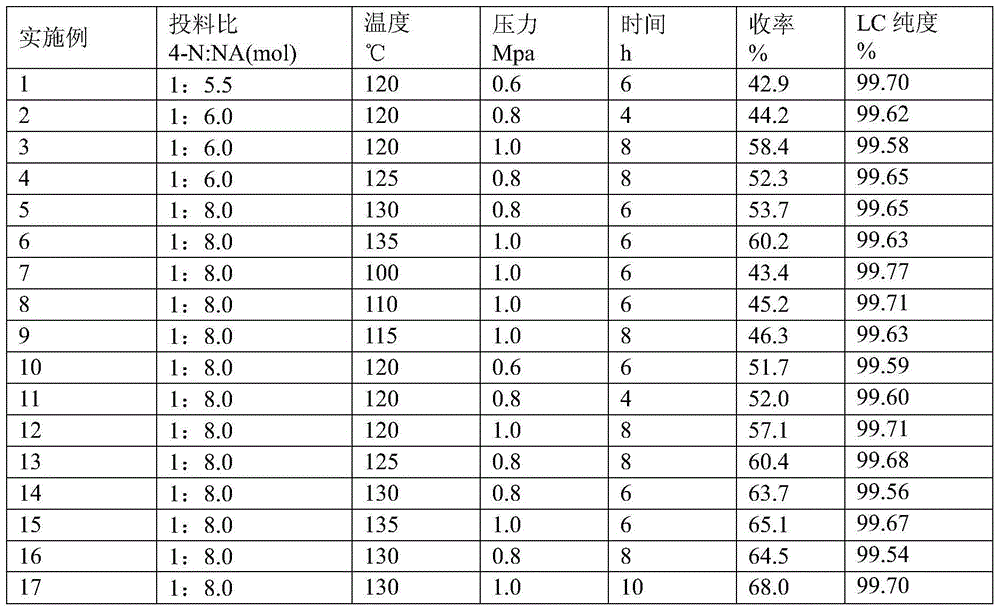
Method for preparing 3-methyl-4-nitrobenzoic acid by oxidizing with nitric acid
InactiveCN104447348AReasonable control of reaction temperatureReasonable control of reaction pressureOrganic chemistryOrganic compound preparationChromium trioxideReaction temperature
The invention relates to a method for preparing 3-methyl-4-nitrobenzoic acid by oxidizing with nitric acid. The method comprises the steps of carrying out oxidation reaction, neutralizing, extracting, decolorizing and acidifying, wherein diluted nitric acid serves as an oxidant, and 2,4-dimethyl-nitrobenzene serves as a basic raw material. The feed ratio, the reaction temperature, the reaction pressure and the reaction time are controlled reasonably, thus the problems of low reaction conversion rate and generation of many by-products are solved. Because the diluted nitric acid serves as the oxidizing agent and replaces potassium permanganate, potassium dichromate, chromium trioxide and other strong oxidizing agents which are high in cost and are difficult to recover, and the generated waste acid can be recycled, the cleanness of industrial synthesis reaction can be improved, and the pollution to environment can be reduced. The method provided by the invention has the advantages that the reaction yield is improved to over 50% from the initial 27%, the reaction condition is mild, the production and operation safety is high, the reaction equipment is simple, and the raw materials including nitric acid and 2,4-dimethyl-nitrobenzene are wide in supply and low in cost and are suitable for large-scale industrial production.
Owner:GANSU YINGUANG CHEM IND GRP CO LTD
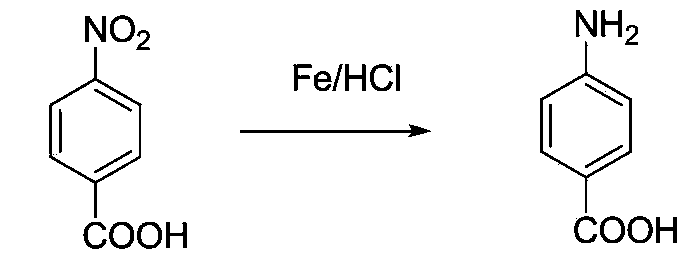
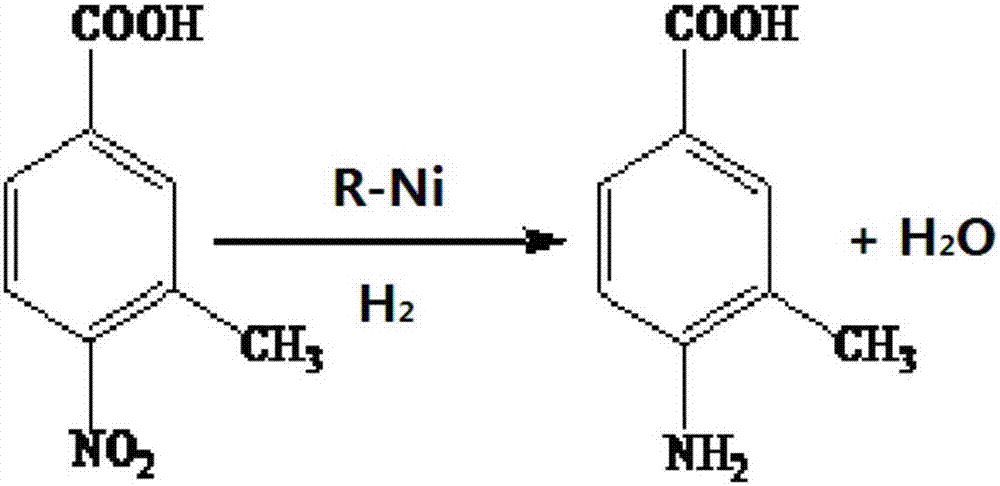
Method for preparing 4-aminobenzoic acid from 4-nitrobenzoic acid by catalytic hydrogenation
InactiveCN104045574AReduce energy consumptionHigh yieldOrganic compound preparationAmino-carboxyl compound preparationP-Aminobenzoic acidSodium salt
The invention relates to the technical field of organic synthetic chemistry, and particularly relates to a method for preparing a 4-aminobenzoic acid from a 4-nitrobenzoic acid by catalytic hydrogenation. 4-aminobenzoic acid is prepared from a sodium salt water solution of 4-nitrobenzoic acid by low-temperature hydrogenation catalytic reduction and acidification under the catalysis of a noble metal catalyst; the selectivity of 4-nitrobenzoic acid can be up to 100%; the yield of 4-aminobenzoic acid can be up to over 96%; the product is white or off-white power; the purity is more than 99% (high performance liquid chromatography (HPLC)). The method has the advantages of short route, fewer three wastes, low energy consumption, high yield and excellent quality. A water solution is prepared from the 4-nitrobenzoic acid and sodium hydroxide; the water solution of the sodium salt is obtained by hydrogenation reduction under the catalysis of a Pd / C catalyst, and then acidified to obtain the 4-aminobenzoic acid. The selectivity of the 4-nitrobenzoic acid can be up to 100%, the yield of 4-aminobenzoic acid can be up to over 96%, the product is white or off-white power, and the purity is more than 99% (HPLC).
Owner:JIANGSU KANGHENG CHEM
Method for preparing tolvaptan intermediate
ActiveCN102690211ASimple processing methodQuality improvementOrganic compound preparationCarboxylic acid amides preparationHydrolysisMethyl group
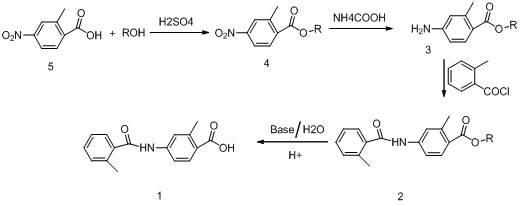
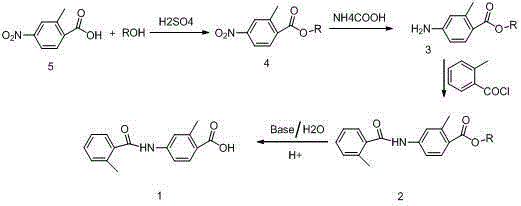
The invention relates to a method for preparing a tolvaptan intermediate. The method comprises the following steps of: reacting 2-methyl-4-nitrobenzoic acid used as an initial raw material and alcohols under the action of a catalyst to generate 2-methyl-4-nitrobenzoate, and reacting under the action of palladium-carbon catalyst by using ammonium formate as hydrogen donor to obtain 2-methyl-4-aminobenzoate compounds; and reacting the 2-methyl-4-aminobenzoate compounds and o-toluoyl chloride under the alkaline condition to generate 2-methyl-4-N-(2-methylbenzoyl)benzoate, adding alkali and a catalyst at presence of water to perform hydrolysis, extracting a water layer by using an organic reagent for one to two times, after layering, collecting a water-layer solution, adjusting the pH of the water-layer solution to be 5 to 6 to separate solid out, filtering an aqueous solution out to obtain solid remnant, and drying to obtain a target product. By the method, the quality and yield of a product are improved; a method for treating the intermediate is simplified; and a simple and feasible process method is provided for industrial production.
Owner:ZHENGZHOU MINGZE MEDICAL TECH
Preparation method of 4-ethyl aminobenzoate powder
ActiveCN107501108AMeeting high demands on granularitySmall granularityOrganic compound preparationAmino-carboxyl compound preparationP-Aminobenzoic acidDodecylsulfonic acid
The invention discloses a preparation method of a 4-ethyl aminobenzoate powder. The preparation method includes the steps of: 1) catalytically hydrogenating 4-nitrobenzoic acid to prepare 4-aminobenzoic acid; 2) esterifying the 4-aminobenzoic acid with ethanol to prepare a 4-ethyl aminobenzoate crude product; 3) performing ethanol recrystallization, in the presence of alkyl sulfonate, to the 4-ethyl aminobenzoate crude product to prepare the 4-ethyl aminobenzoate powder. In the method, a less amount of alkyl sulfonates, such as sodium dodecyl sulfate, is added during the ethanol recrystallization step, so that the crystallization process of the product is controlled well. The 4-ethyl aminobenzoate powder has small granularity and uniform granularity distribution, is high in quality, and can satisfy the high demand on granularity in the fields of medicines, materials and cosmetics.
Owner:CHANGZHOU SUNLIGHT PHARMA
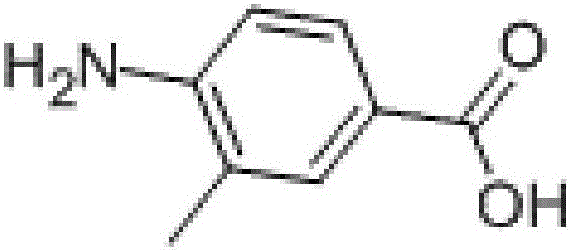
Method for producing 3-methyl-4-aminobenzoic acid through catalytic hydrogenation method
InactiveCN106995382AAvoid pollutionAvoid wastingOrganic compound preparationAmino-carboxyl compound preparationHydrogenation reactionP-Aminobenzoic acid
The invention discloses a method for producing 3-methyl-4-aminobenzoic acid through a catalytic hydrogenation method. According to the method, 3-methyl-4-nitrobenzoic acid is dissolved in an aqueous solution, the obtained solution is added in a hydrogenation kettle, a catalyst is added, hydrogen is led in, a hydrogenation reaction is carried out, and acidification, crystallization, and drying are carried out to obtain 3-methyl-4-aminobenzoic acid. The raw material conversion rate is 100%, and the obtained product is white powder, has the chromatogram purity no less than 99%, and has the yield no less than 95%.
Owner:济南和润化工科技有限公司
Synthesis method of 2-methyl-4-nitrobenzoic acid
InactiveCN103408430AHigh reaction yieldHigh yieldOrganic chemistryOrganic compound preparationSynthesis methodsHigh pressure
The invention discloses a synthesis method of 2-methyl-4-nitrobenzoic acid. The method is used for synthesizing the 2-methyl-4-nitrobenzoic acid by taking 4-dimethylnitrobenzene as a basic raw material and dilute nitric acid as an oxidant. A free radical initiator and a phase transfer catalyst which are added in the reaction process can be used for remarkably increasing the reaction yield and ensuring that the yield of the 2-methyl-4-nitrobenzoic acid as an oxidation product is up to 83.5%. The method disclosed by the invention is mild in reaction condition, capable of avoiding high temperature and high pressure, good in selectivity, high in yield and suitable for engineering application.
Owner:NANJING UNIV OF SCI & TECH
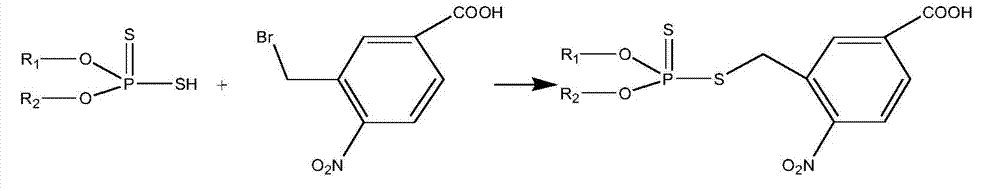
High performance liquid chromatography-tandem mass spectrum detection method of dithiophosphate pesticide metabolites
The invention discloses a high performance liquid chromatography-tandem mass spectrum detection method of dithiophosphate pesticide metabolites, belonging to the detection field of dithiophosphate pesticide metabolites. The method comprises the following steps: carrying out pre-treatment on a sample containing the dithiophosphate pesticide metabolites; carrying out derivatization treatment on the sample by 3-bromo methyl-4-nitrobenzoic acid; and detecting the derivatization products by liquid chromatography-tandem mass spectrum. According to the detection method disclosed by the invention, the recovery rate of the dithiophosphate pesticide metabolites in soil is 79.3-90.7%, the degrees of precision are 9.38% and 5.93%, the detection limits are 0.01mg / kg and 0.005mg / kg, and the linearly dependent coefficients in a range of 0.005-1.000mg / kg are 0.9992 and 0.9997. The method has good recovery rate, limit of quantitation, degree of precision and linearity, and meets the requirements on detection methods of pesticide residues and metabolites thereof in agricultural products.
Owner:黑龙江省华测检测技术有限公司
Preparation method of chemical intermediate benzocaine
InactiveCN106366009AHigh catalytic efficiencyReduced catalytic efficiencyOrganic compound preparationAmino-carboxyl compound preparationRefluxFiltration
The invention discloses a preparation method of chemical intermediate benzocaine. The preparation method of the chemical intermediate benzocaine comprises the steps: S1, adding 4-nitrobenzoic acid, absolute ethanol, a solid catalyst and a water-carrying agent into a reaction container with a water segregator and performing hot reflux for 1 to 6 hours, wherein the solid catalyst takes magnesium aluminum hydrotalcite as a precursor and modified nanometer bentonite as a carrier, the weight of the precursor is 25 to 45 percent that of the carrier, and the solid catalyst is prepared by grinding and mixing the precursor and the carrier uniformly and calcining at 500 to 700 DEG C for 2 to 4 hours; S2, discharging water out of the water segregator, replenishing the absolute ethanol and continuously performing reflux and water segregation until no water drops occur; S3, after the reaction is finished, filtering when the reaction liquid is hot and putting the filtrate into a hydrogenation kettle to perform hydrogenation reaction; S4, after the hydrogenation is finished, performing heat filtration to remove the catalyst from the system, cooling and filtering under the protection of nitrogen, and drying to obtain the chemical intermediate benzocaine. The preparation method of the benzocaine, provided by the invention, is high in conversion rate; the solid catalyst has high catalytic efficiency and can be repeatedly used, and the catalytic efficiency is not obviously reduced after the catalyst is repeatedly used for 50 times.
Owner:安徽金邦医药化工有限公司
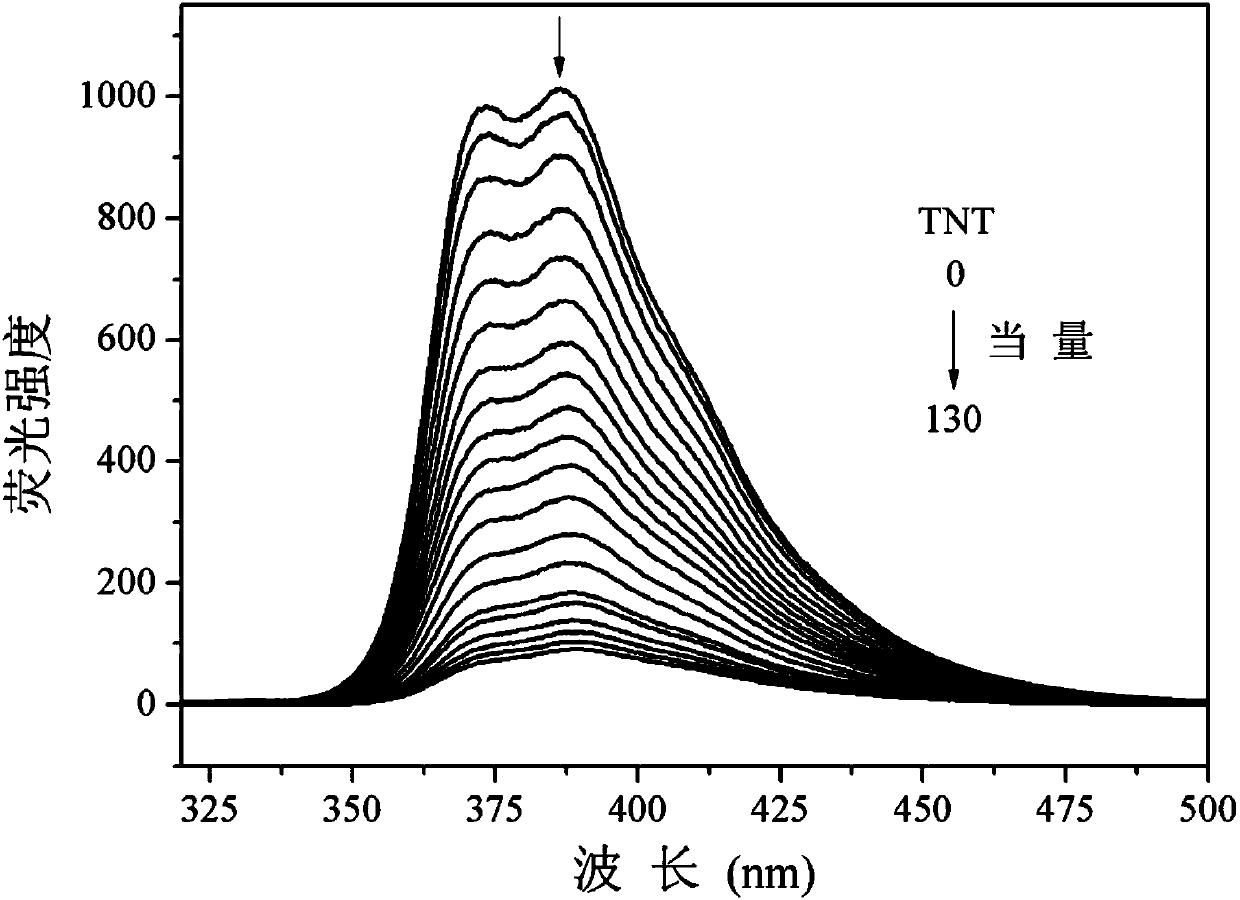
Application of tribenzothiazolyl benzene to nitryl aroma explosive fluorescence detection
ActiveCN107782707AEquivalent lowMicro trace detection is goodFluorescence/phosphorescenceLower limitBenzene
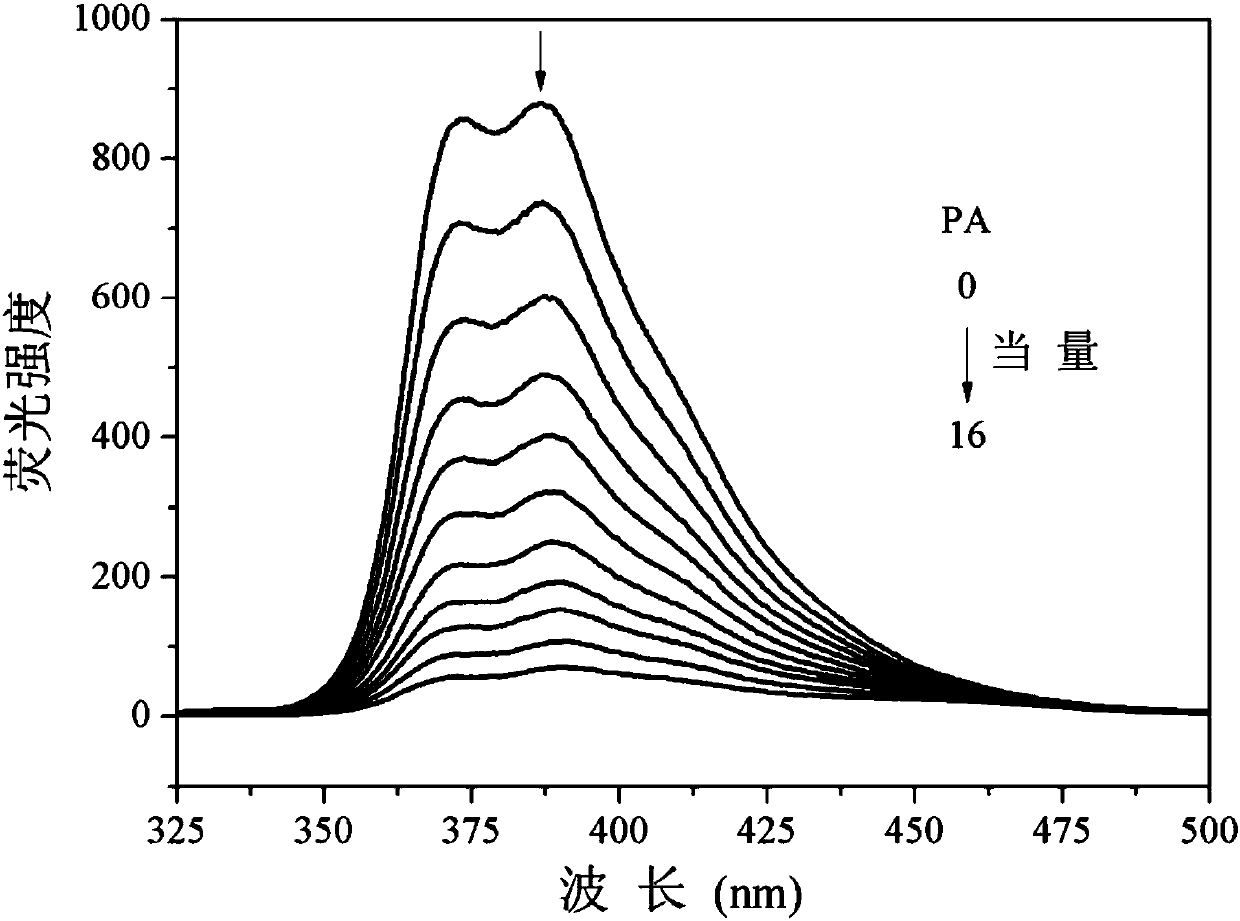
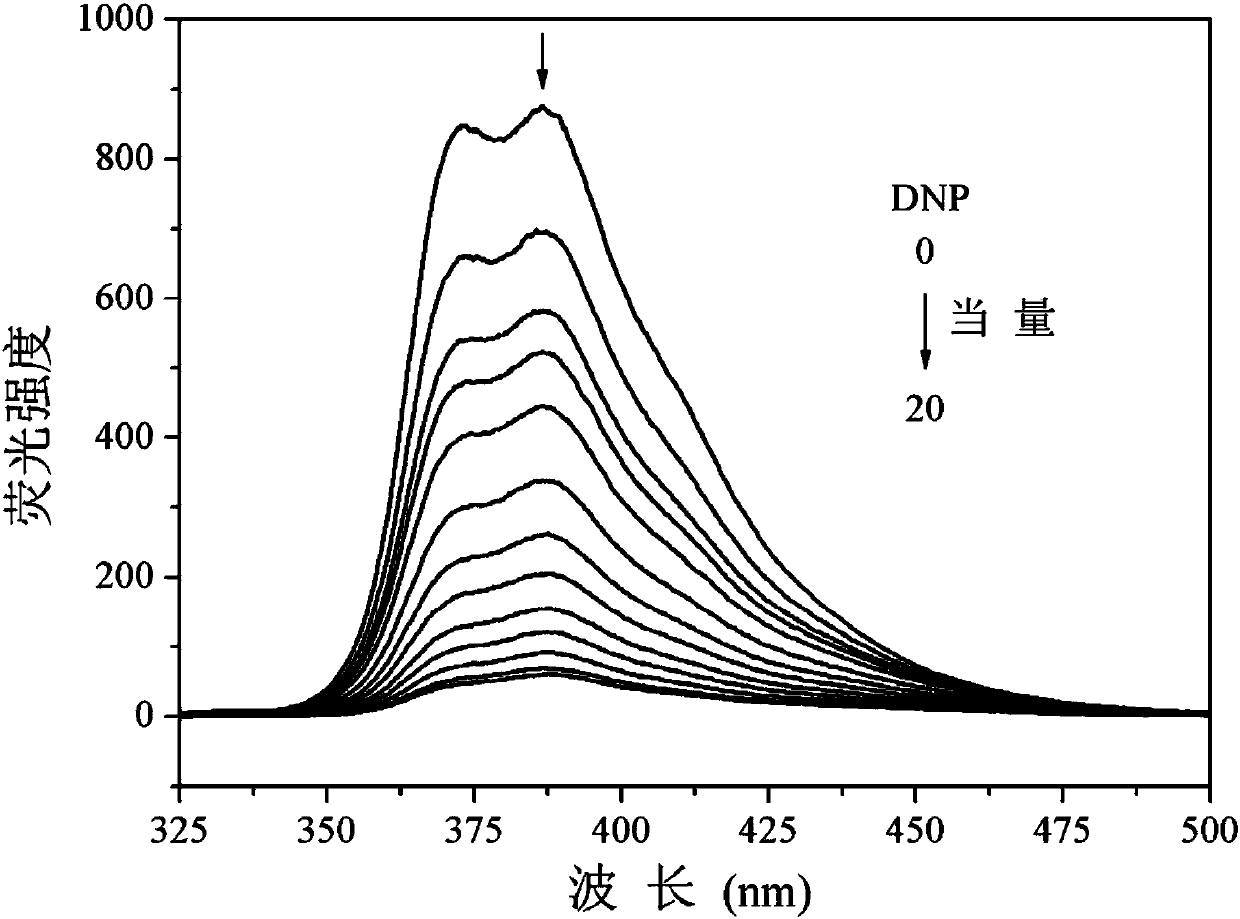
The invention discloses an application of tribenzothiazolyl benzene to nitryl aroma explosive fluorescence detection. According to the application, the tribenzothiazolyl benzene serves as a micro-trace fluorescence detection reagent and is applied to detection of nitryl aroma explosives comprising 2, 4, 6-trinitro-toluene, 2, 4, 6-trinitrophenol, 2, 4-dinitrophenol, 3, 5-dinitrosalicylic acid, 4-nitrophenol, 2, 4-dinitrotoluene, 4-methyl nitrobenzene, 4-nitrobenzaldehyde and nitrobenzene or 4-nitrobenzoic acid, fluorescence of the tribenzothiazolyl benzene can be obviously quenched, the tribenzothiazolyl benzene has good fluorescence detection effects on typical nitryl aroma explosives, equivalent quenched by the tribenzothiazolyl benzene is low by the aid of the detected nitryl aroma explosive, detection lower limit is low, response time is short, response can be achieved within 5 seconds, and micro-trace detection of the nitryl aroma explosives can be effectively performed.
Owner:SOUTH CHINA NORMAL UNIVERSITY +2
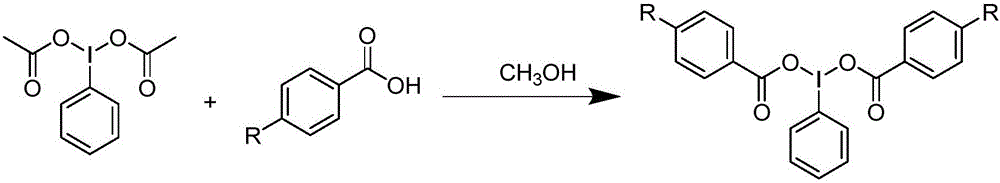
Preparation method and device for iodobenzene dibenzoate derivative
InactiveCN106278899ASimple processMild reaction conditionsOrganic chemistryOrganic compound preparationBenzoic acidPivalic acid
The invention relates to a preparation method and device for an iodobenzene dibenzoate derivative. The method includes the steps that 6 mmol of iodobenzene diacetate and 12 mmol of benzoic acid or a benzoic acid derivative or pivalic acid are added into a 25 mL round-bottom flask first, and 5 ml of methyl alcohol is added till benzoic acid or the benzoic acid derivative and iodobenzene diacetate are completely dissolved in methyl alcohol; the round-bottom flask is placed on a rotary evaporator to be heated to 45 DEG C for a reaction of 0.5 h; reduced pressure distillation is carried out to spin-dry the solvent, and it can be seen that a large amount of white solid matter is separated out; the reaction solution and obtained solid matter are poured into a Buchner funnel, the solid matter is washed with 15 ml of methyl alcohol three times, remaining white solid matter is aired, and the iodobenzene dibenzoate derivative is obtained, wherein the benzoic acid derivative is 4-methoxybenzoic acid or 4-methyl benzoic acid or 4-chlorobenzoic acid or 4-fluorobenzoic acid or 4-nitrobenzoic acid. By means of the preparation method and device, the reaction can be completed in one step, and the technological process is simple; conditions are mild, selectivity is high, environmental friendliness is achieved, and the yield is 72-95%.
Owner:HUBEI UNIV OF TECH
Production method of 3-methyl-4-nitrobenzoate trichloronitrile butyl ester
ActiveCN110105239ALow priceHigh hydroxyl valueCarboxylic acid nitrile preparationOrganic compound preparationCalcium hydroxideAcrylonitrile
The invention belongs to the technical field of fine chemistry, and relates to a production method of 3-methyl-4-nitrobenzoate trichloronitrile butyl ester. The production method comprises following steps: (1), cuprous chloride is taken as a catalyst, trichloroacetaldehyde and acrylonitrile are reacted at 65 to 95 DEG C to prepare 2, 4, 4-trichloro-4-formaldehyde butyronitrile; (2), formaldehyde,calcium hydrate, and 2, 4, 4-trichloro-4-formaldehyde butyronitrile are reacted at 30 to 70 DEG C so as to prepare 2, 4, 4-trichlorocyanbutanol; (3), 3-methyl-4 nitrobenzoic acid, pyridine, and thionyl chloride are reacted at 30 to 120 DEG C to prepare 3-methyl-4-nitro benzoyl chloride; and (4), 2, 4, 4-trichlorocyanbutanol and 3-methyl-4-nitro benzoyl chloride are reacted at 30 to 50 DEG C so asto obtain a finished product. The production method is capable of increasing the entire reaction yield, and reducing production cost; and is suitable for industrialized production.
Owner:山东华科化工有限公司
Method for preparing 3-methyl-4-aminobenzoic acid through catalytic hydrogenation
InactiveCN107501106ARaw materials are easy to getEasy to operateOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisP-Aminobenzoic acid
The invention provides a method for preparing 3-methyl-4-aminobenzoic acid through catalytic hydrogenation, belonging to the technical field of chemical synthesis. The method comprises the following steps: adding 3-methyl-4-nitrobenzoic acid and a solvent into a reaction vessel so as to obtain a 3-methyl-4-nitrobenzoic acid solution, and adjusting the pH value of the solution to be alkaline; pouring the obtained alkaline aqueous solution into an autoclave, adding a catalyst, respectively carrying out replacement with nitrogen and hydrogen for three times, introducing ammonia gas for 2 minutes, carrying out pressurizing with hydrogen to 3.45 MPa to 4.50 MPa, and carrying out a reaction under the pressure of 4.50 to 2.50 MPa at 100 to 160 DEG C so as to obtain a reaction solution; and subjecting the reaction solution to post-processing so as to obtain the 3-methyl-4-aminobenzoic acid. The method provided by the invention has the advantages of easily-available raw materials, simple operation, low production amount of three wastes, environmental friendliness, capability of reaching a mole yield up to 90%, and more applicability to industrial production.
Owner:中涛新材料有限公司
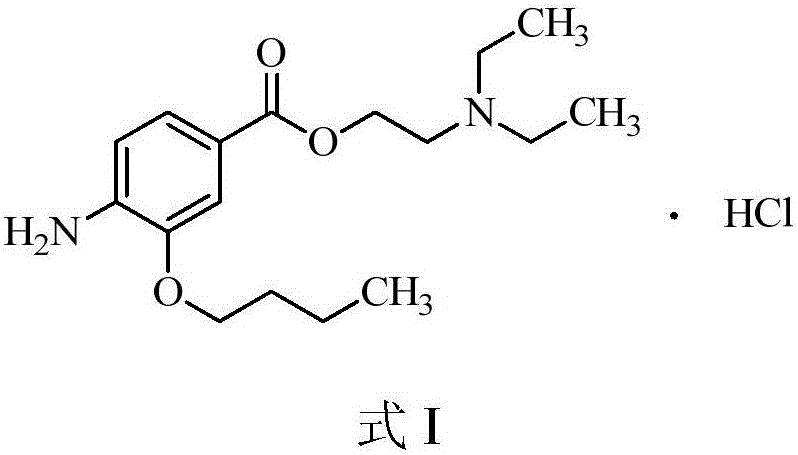
Preparation method of oxybuprocaine hydrochloride
InactiveCN106810463ALow costOrganic compound preparationAmino-carboxyl compound preparationHydrazine compoundHigh pressure
The invention discloses a preparation method of oxybuprocaine hydrochloride. The preparation method includes following steps: in the presence of alkali I, subjecting 3-hydroxy-4-ethyl nitrobenzoate and bromobutane to electrophilic substitution reaction to obtain 3-butoxy-4-ethyl nitrobenzoate; under alkaline condition, enabling 3-butoxy-4-ethyl nitrobenzoate to be in hydrolysis reaction, and adjusting pH value of a system after hydrolysis reaction to 1-3 to obtain 3-butoxy-4-nitrobenzoic acid; in the presence of alkali II, subjecting the 3-butoxy-4-nitrobenzoic acid and diethylamino ethyl chloride to electrophilic substitution reaction to obtain 4-nitro-2-butoxy nitrobenzoic acid-2-(diethylamino) ethyl ester, and subjecting the 4-nitro-2-butoxy nitrobenzoic acid-2-(diethylamino) ethyl ester to reduction reaction under action of ferric trichloride and hydrazine hydrate to obtain oxybuprocaine hydrochloride. 3-hydroxy-4-ethyl nitrobenzoate is adopted as a raw material to obtain a target product through four steps of conventional reaction; each step of reaction does not need high-pressure condition, and needed raw materials are all conventional compounds, so that the preparation method is easy to obtain and low in cost.
Owner:SHENZHEN OASIS PHARMA
Compound with antidepressant activity and preparation method thereof
InactiveCN109928909AGood antidepressant activityNew molecular structureNervous disorderOrganic chemistryStructural formulaChromatographic column
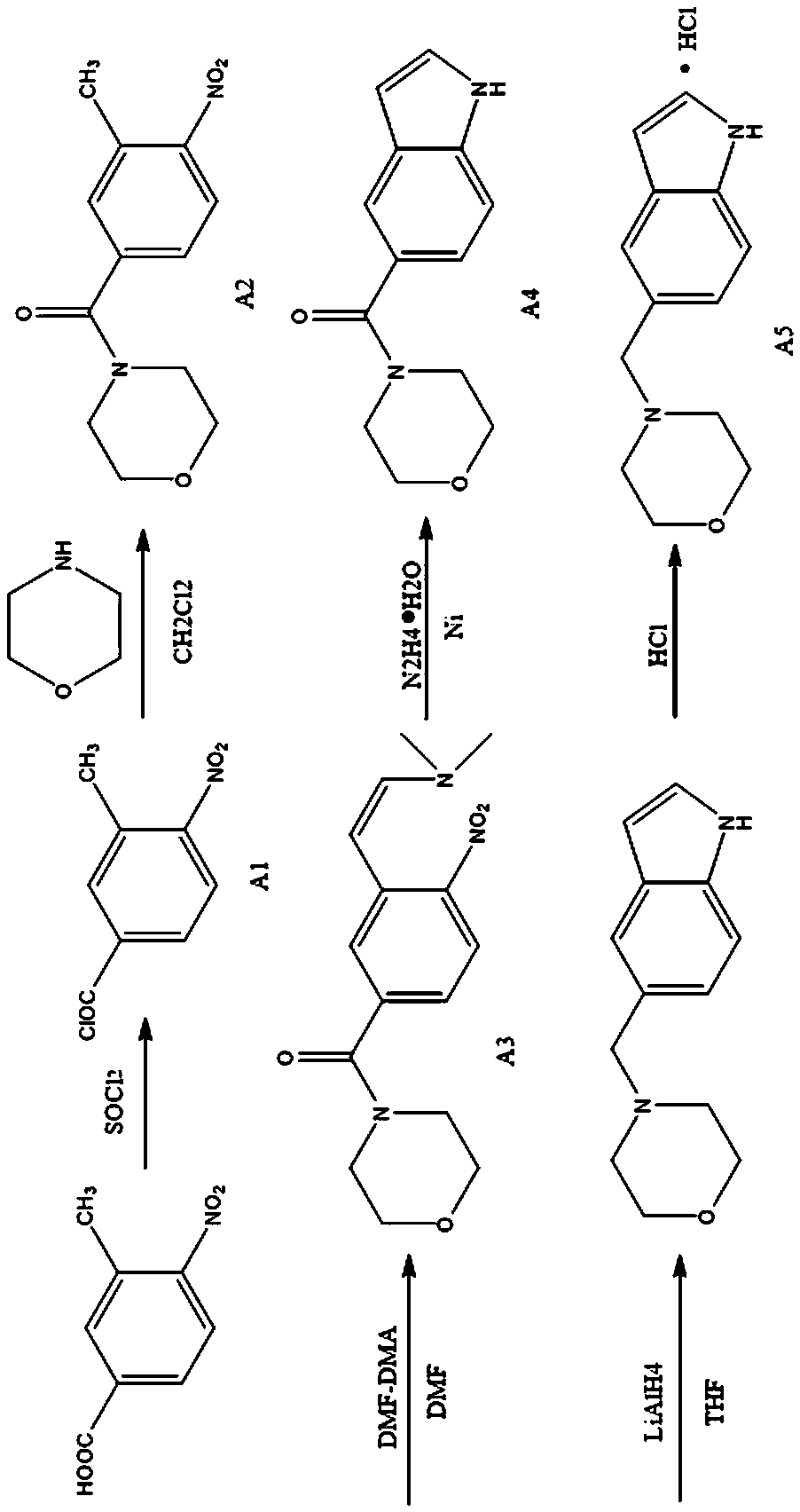
The present invention provides compounds with antidepressant activity and a preparation method thereof. The molecular formula of the compound is C13H17NO2Cl, the molecular weight is 252.5, the meltingpoint is 207 DEG C, and the structural formula is shown in the specification. The preparation method comprises the following steps: adding 3-methyl-4-nitrobenzoic acid and thionyl chloride into a first reaction container, stirring, dissolving and uniformly mixing the mixture, and condensing and refluxing the mixture at the temperature of 80 + / -3 DEG C to obtain acyl chloride A1; dropwise adding the acyl chloride A1, cooled to 0 DEG C, into a second reaction container to obtain an intermediate product A2; dissolving A2 in DMF, and adding the solution into a third reaction container to obtain an intermediate product A3; adding the obtained intermediate product A3 into a fourth reaction container to obtain a crude product, and treating the crude product in a chromatographic column to obtainan intermediate product A4; and adding the obtained intermediate product A4 into a sixth reaction container to obtain a powdery solid A5, which is the compound with the antidepressant activity.
Owner:NANHUA UNIV
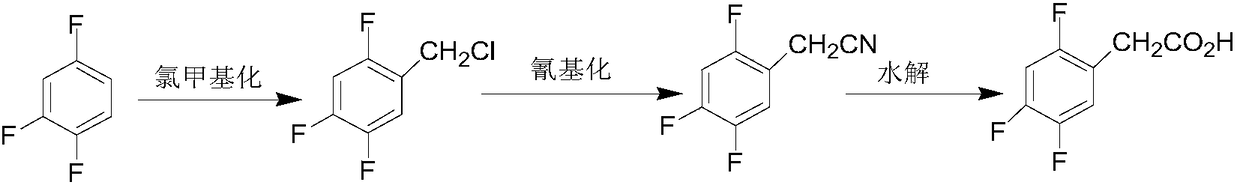
Preparation method of 2, 5-difluoro-4-nitrobenzoic acid
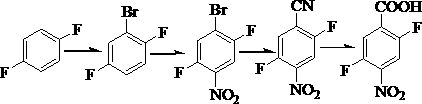
The invention discloses an industrial preparation method of 2, 5-difluoro-4-nitrobenzoic acid. According to the industrial preparation method of the 2, 5-difluoro-4-nitrobenzoic acid, p-difluorobenzene serves as an initial raw material, and the 2, 5-difluoro-4-nitrobenzoic acid is synthesized by bromination, nitration, cyaniding and hydrolysis four-step reaction. The 2, 5-difluoro-4-nitrobenzoic acid obtained in the process is off-white powdery solid with the purity of 98.5%, raw material conversion rate in each step reaches 100%, and the total yield of the whole process reaches 44.6%.
Owner:CHANGZHOU UNIV
The preparation method of tolvaptan intermediate
ActiveCN102690211BSimple processing methodQuality improvementOrganic compound preparationCarboxylic acid amides preparationMethyl groupHydrolysis
The invention relates to a method for preparing a tolvaptan intermediate. The method comprises the following steps of: reacting 2-methyl-4-nitrobenzoic acid used as an initial raw material and alcohols under the action of a catalyst to generate 2-methyl-4-nitrobenzoate, and reacting under the action of palladium-carbon catalyst by using ammonium formate as hydrogen donor to obtain 2-methyl-4-aminobenzoate compounds; and reacting the 2-methyl-4-aminobenzoate compounds and o-toluoyl chloride under the alkaline condition to generate 2-methyl-4-N-(2-methylbenzoyl)benzoate, adding alkali and a catalyst at presence of water to perform hydrolysis, extracting a water layer by using an organic reagent for one to two times, after layering, collecting a water-layer solution, adjusting the pH of the water-layer solution to be 5 to 6 to separate solid out, filtering an aqueous solution out to obtain solid remnant, and drying to obtain a target product. By the method, the quality and yield of a product are improved; a method for treating the intermediate is simplified; and a simple and feasible process method is provided for industrial production.
Owner:ZHENGZHOU MINGZE MEDICAL TECH
Preparation of 2-ethoxy-4-acetaminobenzoic acid methyl ester
InactiveCN101348444ALow priceRaw materials are easy to getOrganic compound preparationCarboxylic acid amides preparationIce waterPolymer science
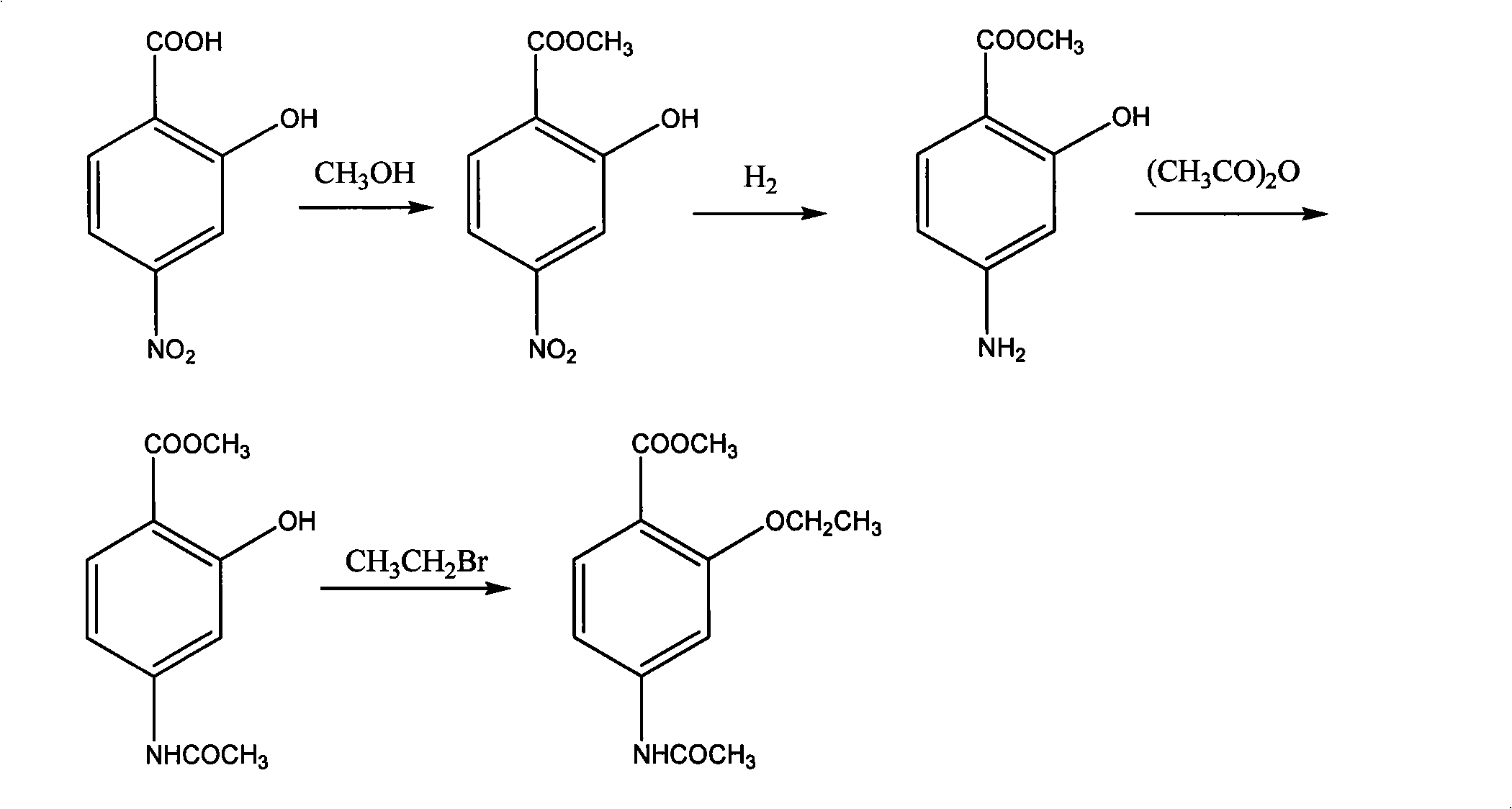
A preparation method for 1.2-oxethyl-4-acetamido methyl benzoate comprises the following steps that 2-hydroxyl-4-nitrobenzoic acid is taken as a raw material, is dissolved in 4 to 5mol of methanol as calculated by raw material per mol and is added with 5 to 10g of catalyst; the mixture is heated and undergoes back flow; when reaction is carried out for 3 hours, the catalyst is filtered out; hydrogenation reduction of nitro is carried out under a pressure of 0.3MPa and at a temperature of 50 DEG C; the temperature is controlled between 25 and 35 DEG C; according to a proportion that 1mol acetic anhydride is dripped into every mol of the raw material, acetic anhydride is dripped and stirred at a temperature of between 40 and 50 DEG C for 40min; the mixture is cooled down to room temperature after solidifying; according to a proportion that 200 to 300ml of water is added in every mol of the raw material, water is added and stirred, and acetylated solid can be obtained after sucking filtration and drying; the acetylated solid product obtained from each mol of raw material is dissolved in 100ml of DMF, and 1mol of potassium carbonate and 1mol of CH3CH2Br are added and stirred at a temperature of between 50 and 60 DEG C for 3 hours; the reactants are poured with ice water so as to obtain a brown solid after filtration; normal hexane and toluene with the volume ratio equal to 1 to 1 are used to dissolve the brown solid; and after heated back flow and recrystallization, a silvery white 1.2-oxethyl-4-acetamido methyl benzoate solid can be obtained.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
Synthesis method for 3-formyl-4-methyl nitrobenzoate
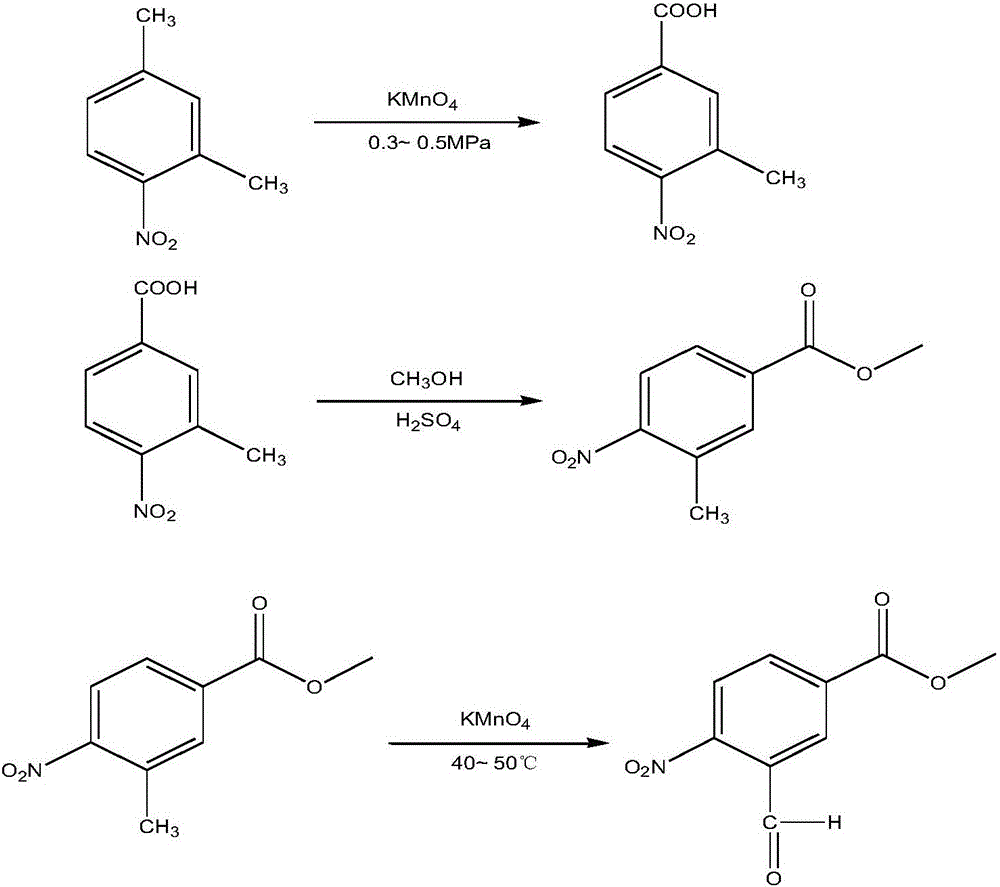
InactiveCN105753708AOrganic chemistryOrganic compound preparationSynthesis methods4-Nitrobenzoic acid
The invention relates to a synthesis method for a compound, in particular to a synthesis method for 3-formyl-4-methyl nitrobenzoate.According to synthesis method, a simple and cheap preparation method for 3-formyl-4-methyl nitrobenzoate is supplied, the reaction conditions in the preparation process are mild, and the yield is high.
Owner:CHANGZHOU UNIV
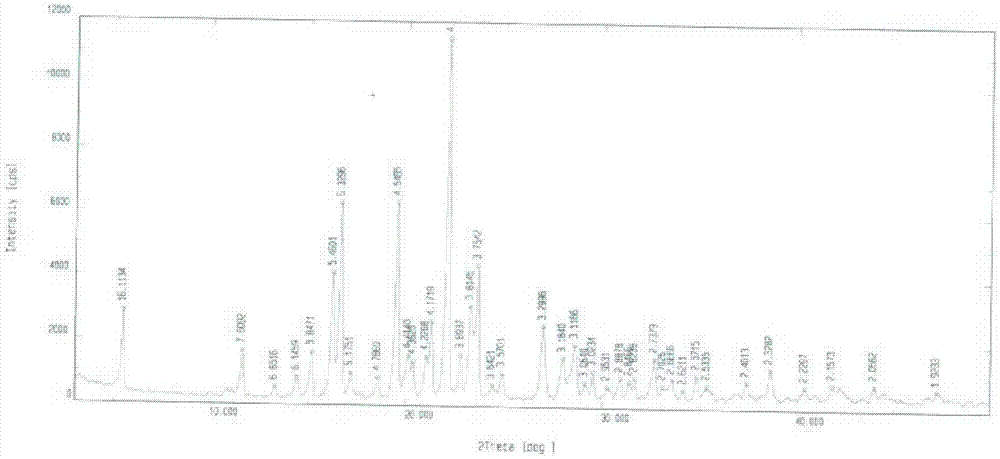
Lixivaptan crystal form I and preparation method and use thereof
The invention belongs to the field of anti heart failure drugs, and more specifically, relates to lixivaptan crystal form I and a preparation method and use of a pharmaceutical composition containing the lixivaptan crystal form I in preparation of drugs for treating hyponatremia. The lixivaptan crystal form I is prepared from 2-chloro-4-nitro benzoic acid as a starting material by esterification, hydrogenation reduction, acylation, hydrolysis, acyl chlorination and other reaction steps, and the prepared lixivaptan purity is 97.5%. The lixivaptan crystal form I and its powder are characterized by X ray diffraction diagrams, and the lixivaptan crystal form I is important for obtaining of compounds with high purity, very determined crystal form and good reproducibility due to good prospects for development and pharmaceutical value of the lixivaptan crystal form I.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of 3-methyl-4-aminobenzoic acid
InactiveCN106831460AFully contactedIncrease reaction rateOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisActivated carbon
The invention provides a preparation method of 3-methyl-4-aminobenzoic acid and belongs to the technical field of chemical synthesis. According to the method, 3-methyl-4-nitrobenzoic acid and a quaternary ammonium salt type phase-transfer catalyst are added to a reaction device, a solvent is added, the mixture is stirred uniformly, reduced iron powder and protonic acid are added to react, and a reaction liquid is obtained; then the reaction device is cooled, sodium carbonate and activated carbon are added for decoloration, the PH value is adjusted to be alkaline, iron mud is filtered and washed twice with a sodium carbonate solution, a filtered stock and cleaning fluids are mixed, and a light yellow solution is obtained; acid is added to the light yellow solution until the PH value is adjusted to be slightly acid, off white precipitates are separated out, filtered, washed with water and dried, and 3-methyl-4-aminobenzoic acid is obtained. The preparation method is simple, low in cost and more suitable for industrial production, and the product yield reaches 90.1% at most.
Owner:中涛新材料有限公司
3-Hydroxyl-4-nitrobenzoic acid and preparation method thereof
InactiveCN105669462AHigh purityGood removal effectOrganic compound preparationNitro compound preparationNitrationMethyl group
The purpose of this invention is to provide a kind of 3-hydroxyl-4-nitrobenzoic acid and preparation method thereof, take cheap and easy-to-get m-cresol as raw material, first nitration reaction, then carry out oxidation reaction. with H 2 o 2 As an oxidizing agent, the methyl group is oxidized to a carboxyl group, thereby producing 3-hydroxyl-4-nitrobenzoic acid. The method has the advantages of low raw material cost, simple operation, few by-products, high yield, mild reaction environment conditions, and is suitable for large-scale industrial production.
Owner:叶芳
Method for preparing 4-aminobenzoic acid by catalytic hydrogenation
InactiveCN104326928AGood workmanshipOperational securityOrganic compound preparationAmino-carboxyl compound preparationP-Aminobenzoic acidSolvent
The invention relates to the field of production of 4-aminobenzoic acid and specifically relates to a method for preparing 4-aminobenzoic acid by catalytic hydrogenation. The method comprises the following steps: preparing a water solution of a neutral sodium salt from 4-nitrobenzoic acid and the equal molar amount of an alkali (sodium hydroxide) for preparing 4-nitrobenzoate by using 3 times the mass of solvent water the mass of which is 3 times that of 4-nitrobenzoic acid and alkali, performing hydrogenation reduction for 2h by taking 5% Pd / C as a catalyst, controlling the pressure of hydrogen to 1-2MPa and controlling the temperature to 60-70 degrees till the pressure is not reduced basically to obtain the water solution of the sodium salt, further acidifying and filtering to obtain white 4-aminobenzoic acid, wherein the yield is above 95% and the purity is above 99%. The method provided by the invention has the advantages of perfect process, low energy consumption, short reaction period and no corrosion and is safe to operate, and the environment-friendly process for preparing 4-aminobenzoic acid by taking 4-nitrobenzoic acid as a raw material and performing catalytic hydrogenation at low temperature and low pressure is high in production efficiency.
Owner:江苏恒祥化学股份有限公司
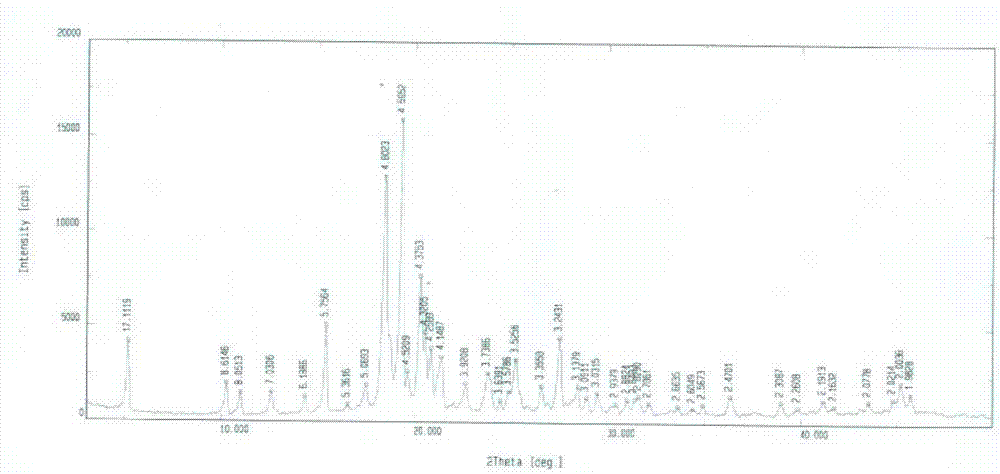
Lixivaptan crystal form II, preparation method and use thereof
The invention belongs to the field of anti-heart failure drugs, and more particularly relates to a lixivaptan crystal form II and a preparation method thereof, a drug composition containing the lixivaptan crystal form II, and a use of the lixivaptan crystal form II in preparation of hyponatremia treatment drugs. According to the present invention, 2-chloro-4-nitrobenzoic acid is adopted as a starting raw material, and esterification, hydrogenation reduction, acylation, hydrolysis, chlorination and other reaction steps are performed to prepare the lixivaptan crystal form II, wherein the purity of the obtained lixivaptan is 97.5%, and the lixivaptan crystal form II is characterized by the powder X-ray diffraction pattern; and due to the good development prospects and the pharmaceutical values of the compound, it is important to obtain the compound with characteristics of high purity, determined crystal form and good reproducibility.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com