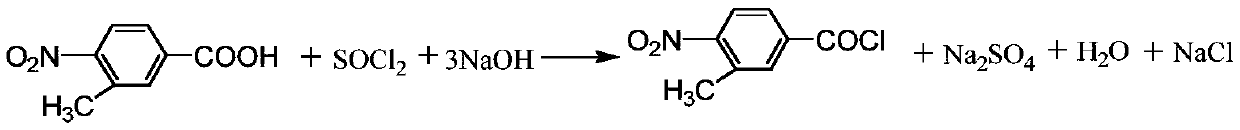
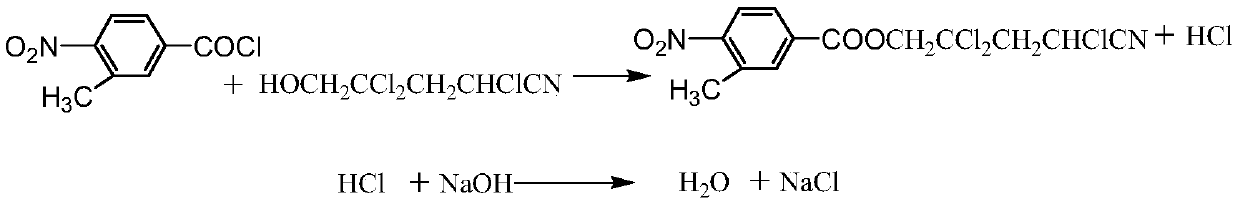
Production method of 3-methyl-4-nitrobenzoate trichloronitrile butyl ester
A technology of trichloronitrile butyl nitrobenzoate and nitrobenzoic acid is applied in the preparation of carboxylic acid nitrile, chemical instruments and methods, preparation of organic compounds, etc., and can solve difficult separation, low product purity, and many impurities. and other problems to achieve the effect of reducing production cost and improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Preparation of condensation unit 2,4,4-trichloro-4-formaldehyde solution butyronitrile
[0031] Enamel reaction kettle R1050, R1051, R1052, R1053, R1054, R1055, add chloral 1000Kg, acrylonitrile 600Kg, catalyst cuprous chloride 50Kg, heat up to 78~82℃, reflux for 24 hours for condensation reaction, after the reaction Cool to 35-40°C, the materials in R1050, R1051 and R1052 are transported to R1031, R1032 or R1034 through R1033, the materials in R1053, R1054 and R1055 are transported to R1035, R1036 or R1037 through R1038, and unreacted is recovered by vacuum distillation A mixture of acrylonitrile and chloral, repeated.
[0032] When the temperature rises to 100°C, the recovery is over, the temperature is lowered to 30°C, 1000Kg of sulfolane is added respectively, the materials in the kettle are transferred to R1003, R1004 and R1005, and the low boilers are further distilled to R1006 and R1007.
[0033] Cool the remaining materials in R1003, R1004 and R1005 to 30°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com