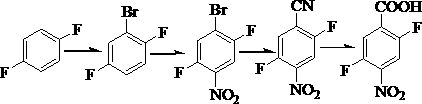
Preparation method of 2, 5-difluoro-4-nitrobenzoic acid
A technology of nitrobenzoic acid and difluorobenzene, which is applied in the field of preparation of 2,5-difluoro-4-nitrobenzoic acid, and can solve problems such as unseen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 2
[0016] (1) Bromination reaction: Add 1000 g of p-difluorobenzene and 5000 mL of sulfuric acid into the reaction vessel, and control the temperature of the water bath to 30 °C. 1620 g of N-bromosuccinimide were added slowly with stirring. During the addition, the temperature should not exceed 40°C. After the addition, the reaction was incubated for 1 h, followed by GC until the end of the reaction. After the reaction, the reactant was poured into ice water to precipitate a solid, which was suction filtered to obtain 1568 g of light yellow powdery solid 2,5-difluorobromobenzene with a content of 98% and a yield of 92.7%.
[0017] (2) Nitration reaction: Add 1208 g of 2,5-difluorobromobenzene to the reaction vessel, add 5000 mL of concentrated sulfuric acid, control the temperature in an ice-water bath to about 5 degrees, then add 462 g of potassium nitrate, and keep warm for 1 h, GC trace until the end of the reaction. Water analysis in ice water, suction filtration, and dryi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com