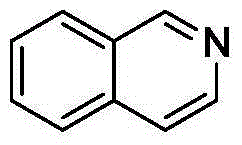
Preparation method of 3-chloro(bromo)-6-nitroisoquinoline
A technology of nitroisoquinoline and nitrobenzoic acid, which is applied in the field of preparation of 3-chloro-6-nitroisoquinoline, can solve problems such as inability to promote
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
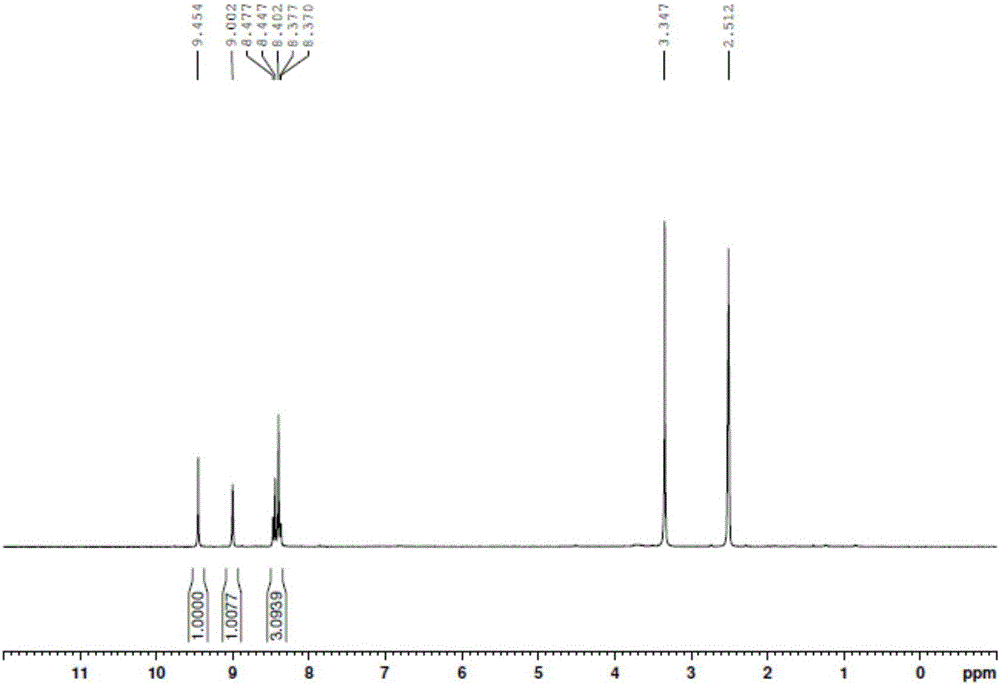
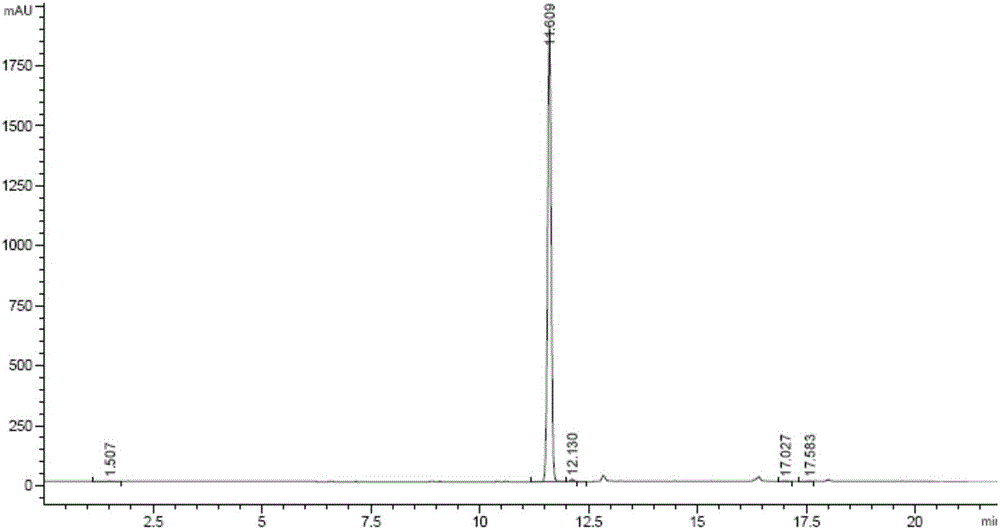
Image
Examples
Embodiment 1
[0039] The 3-chloro-6-nitroisoquinoline of the present embodiment is prepared by the following method:
[0040] A kind of 3-chloro-6-nitroisoquinoline preparation method is characterized in that comprising the steps:
[0041]S1. First add 200g of 2-chloro-4-nitrobenzoic acid into 2000ml of dimethyl malonate, then inject nitrogen into the liquid surface for about 1 hour, then add 2g of cuprous bromide and 117g of sodium methoxide, Keep nitrogen, then raise the temperature to 90-100°C and react for 16 hours. After TLC monitors the reaction is complete, cool to room temperature, add 2L water and 2L petroleum ether, wash the water phase with 500ml petroleum ether and 500ml toluene respectively, and then wash the water phase with 2N HCl Adjust the pH to 2-3, filter the precipitated solid with suction, and obtain most of the product, about 160 g. The aqueous phase was further extracted with 500 mL of petroleum ether, and the organic phases were combined, washed with saturated NaCl,...
Embodiment 2
[0052] The 3-bromo-6-nitroisoquinoline of the present embodiment is prepared by the following method:
[0053] A kind of 3-bromo-6-nitroisoquinoline preparation method is characterized in that comprising the steps:
[0054] S5. 50 g of 6-nitroisoquinoline-1,3(2H,4H)-dione prepared in step S4 of Example 1 was dissolved in 250 ml of 1,4-dioxane, and 348 g of phosphorus oxybromide was added , react at 105-110°C for 3.5 hours, cool, pour into a beaker, add water dropwise to quench the reaction, and complete the addition in about 30 minutes; adjust the pH to 10 with ammonia water, solids precipitate, filter with suction, and dry the filter cake. The filtrate is extracted with ethyl acetate, and the extract is spin-dried and the dried solid is purified with a silica gel column. The silica gel column uses a mixed solution of petroleum ether and ethyl acetate as the eluent, and the volume ratio of the two is petroleum ether: ethyl acetate Ester=3:1, the product 1,3-dibromo-6-nitroiso...
Embodiment 3
[0059] The 3-chloro-6-nitroisoquinoline of the present embodiment is prepared by the following method:
[0060] A kind of 3-chloro-6-nitroisoquinoline preparation method is characterized in that comprising the steps:
[0061] S1. First add 100g of 2-chloro-4-nitrobenzoic acid to 1000ml of dimethyl malonate, then let the nitrogen gas go deep into the liquid surface for about 2 hours, then add 1g of cuprous bromide and 58.5g of sodium methoxide , keep nitrogen, then raise the temperature to 90-100°C for 12 hours, after TLC monitors the reaction is complete, cool to room temperature, add 1L of water and 1L of petroleum ether, wash the water phase with 250ml of petroleum ether and 250ml of toluene respectively, and then wash the water phase with Adjust the pH to 2-3 with 2N HCl, and filter the precipitated solid with suction to obtain most of the product, about 80 g. The aqueous phase was further extracted with 250 mL of petroleum ether, the organic phases were combined, washed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com