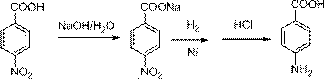Method for preparing 4-aminobenzoic acid by catalytic hydrogenation
An aminobenzoic acid, catalytic hydrogenation technology, applied in chemical instruments and methods, preparation of cyanide reaction, preparation of organic compounds, etc., can solve the problems of long process route, low significance of industrialized production, etc., and achieve perfect process , the effect of high production efficiency and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] 50g 4-nitrobenzoic acid and 12g sodium hydroxide, 150g water are mixed with the aqueous solution of 4-nitrobenzoic acid sodium salt, join in the 1L autoclave, and add 0.5g 5%Pd / C, after nitrogen replacement three times, Hydrogen was replaced twice, the pressure was added to 2 MPa, the temperature was controlled at 60-70 degrees, and the reaction was carried out until the pressure did not drop substantially. After about 2 hours, the catalyst was recovered. The resulting reaction solution was acidified with concentrated hydrochloric acid to pH 3, filtered and washed with water to obtain 4-aminobenzoic acid as a white solid, and 38.9 g of the product was obtained after drying, with a yield of 95% and a purity of 99.5% (HPLC).
[0025]
example 2
[0027] 100g 4-nitrobenzoic acid and 24g sodium hydroxide, 300g water are mixed with the aqueous solution of 4-nitrobenzoic acid sodium salt, join in the 1L autoclave, and add 1.0g 5%Pd / C, after nitrogen replacement three times, Hydrogen was replaced twice, the pressure was added to 2 MPa, the temperature was controlled at 60-70 degrees, and the reaction was carried out until the pressure did not drop substantially. After about 2 hours, the catalyst was recovered. The resulting reaction solution was acidified to pH 3 with concentrated hydrochloric acid, filtered and washed with water to obtain 4-aminobenzoic acid as a white solid, and 78.8 g of the product was obtained after drying, with a yield of 96.1% and a purity of 99.4% (HPLC).
[0028]
example 3
[0030] 150g 4-nitrobenzoic acid and 36g sodium hydroxide, 450g water are mixed with the aqueous solution of 4-nitrobenzoic acid sodium salt, join in the 1L autoclave, and add 1.5g 5%Pd / C, after nitrogen replacement three times, Hydrogen was replaced twice, the pressure was added to 2 MPa, the temperature was controlled at 60-70 degrees, and the reaction was carried out until the pressure did not drop substantially. After about 2 hours, the catalyst was recovered. The resulting reaction solution was acidified with concentrated hydrochloric acid to pH 3, filtered and washed with water to obtain 4-aminobenzoic acid as a white solid, and 118.5 g of the product was obtained after drying, with a yield of 96.3% and a purity of 99.6% (HPLC).
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com