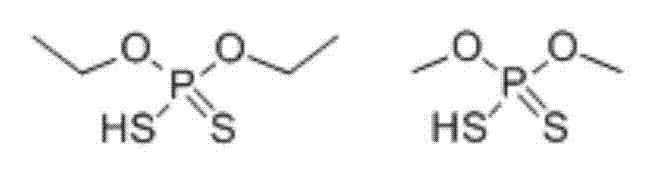High performance liquid chromatography-tandem mass spectrum detection method of dithiophosphate pesticide metabolites
A technology of high performance liquid chromatography and diethyl phosphorodithioate, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of inability to retain, long derivation time, low sensitivity, etc., and achieve the effect of good recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
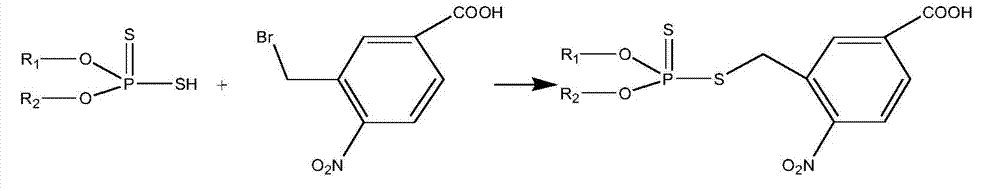
[0028] Example 1 O, O-dimethyl phosphorodithioate and O, O-diethyl phosphorodithioate content in soil Establishment of high performance liquid chromatography-tandem mass spectrometry detection method
[0029] 1. Experimental method
[0030] 1.1. Sample pretreatment and derivation
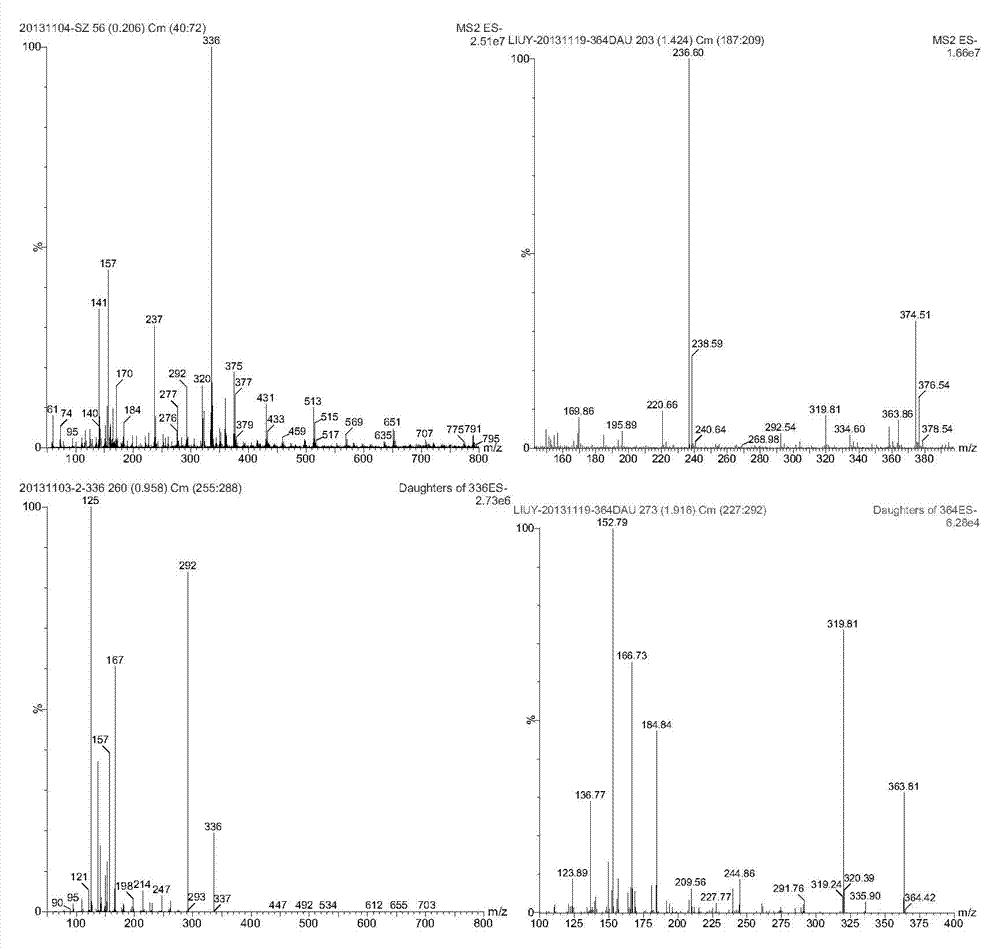
[0031] After the soil sample is air-dried, pass through a 40-mesh sieve, weigh about 5g (accurate to 0.01g) into a 50mL centrifuge tube, add 20mL of acetone, vortex and mix well, extract with an oscillator for 10min, then centrifuge at 4000r / min for 5min, and take The supernatant and the residue were extracted once, and the extracts were combined, evaporated to near dryness, dissolved in 2mL ethyl acetate, passed through 500mg6mL activated carbon and 500mg6mL florisil in series solid-phase extraction column (the small column was activated by 5mL ethyl acetate ), wash the sample vial twice with 1mL ethyl acetate, pass through the series column, then elute the solid-phase extraction column with 5mL e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com