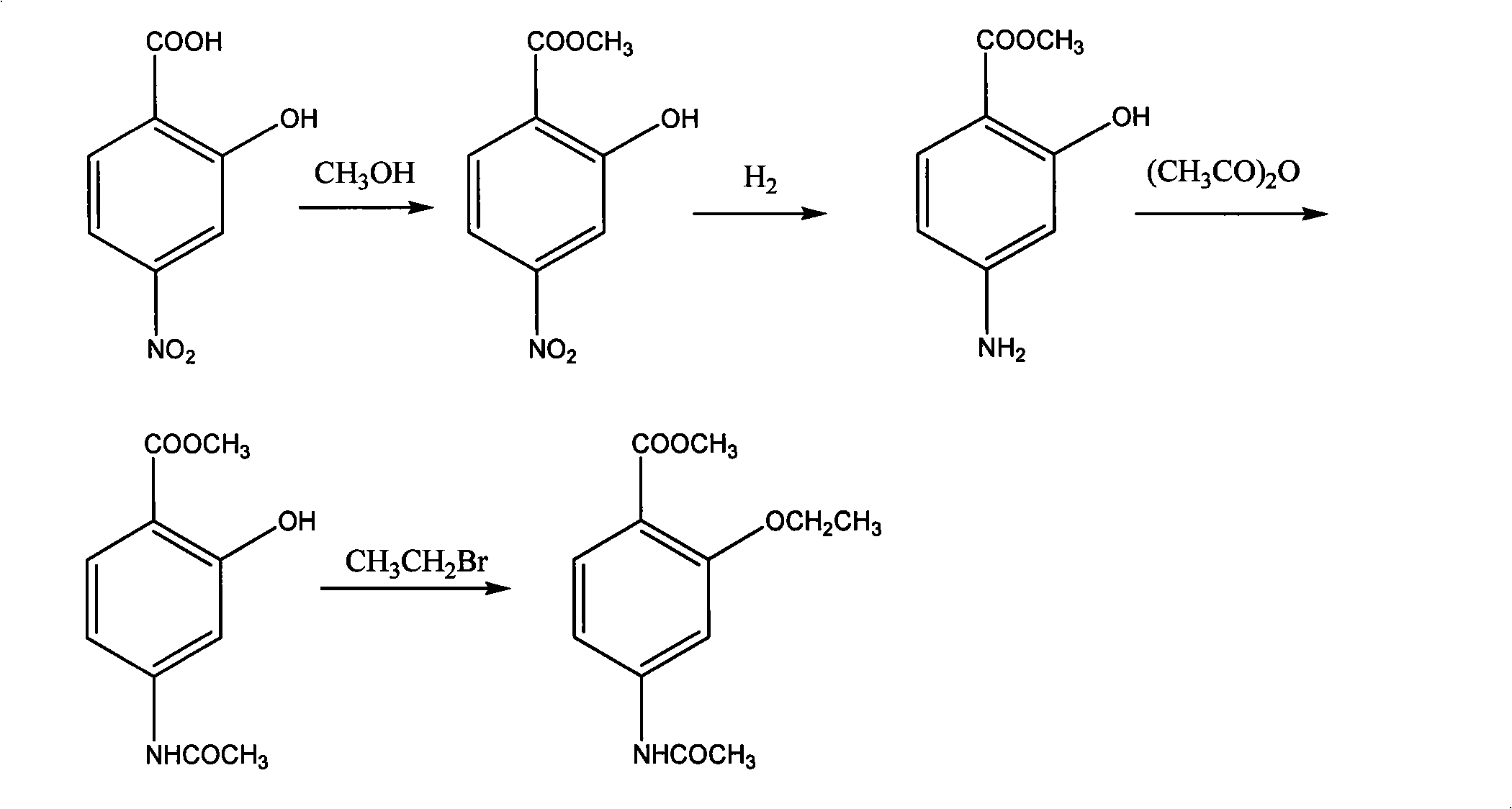
Preparation of 2-ethoxy-4-acetaminobenzoic acid methyl ester
The technology of methyl acetamidobenzoate and methyl nitrobenzoate is applied in the field of preparation of veterinary drug 2-ethoxy-4-acetamidobenzoic acid methyl ester, and can solve the problem that diethyl sulfate is highly toxic, Methyl iodide is expensive, reduces industrial feasibility and other problems, and achieves the effects of feasible process route, low price and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) 2-hydroxyl-4-nitrobenzoic acid reacts with methanol: promptly at room temperature, 1mol 2-hydroxyl-4-nitrobenzoic acid is dissolved in 4-5mol methyl alcohol, adds 5-10g strongly acidic ion exchange resin ( Amberlyst 15), warming up to reflux temperature, reacting for 3 hours, directly filtering to remove the catalyst after esterification, to obtain the filtrate, its content was determined by liquid chromatography to be 99.65% (calculated by external standard method), and the yield was 98.3%.
[0023] (2) hydrogenation reduction of methyl 2-hydroxyl-4-nitrobenzoate: the above-mentioned filtrate is placed in an autoclave, 5-10g Raneynickel is added, and the nitro group is hydrogenated and reduced at 0.3MPa and 50°C, and the reaction reaches the end point ( Sampling for TLC determination) and filtration to remove the catalyst to obtain the filtrate. The content determined by liquid chromatography is 99.76% (calculated by external standard method), and the yield is 99.5...
Embodiment 2
[0028] (1) 2-hydroxyl-4-nitrobenzoic acid reacts with methanol: at room temperature, 1mol 2-hydroxyl-4-nitrobenzoic acid is dissolved in 4-5mol methanol, 5-10g 98% concentrated sulfuric acid is added, and the temperature rises To the reflux temperature, reacted for 3 hours, directly filtered to remove the catalyst after esterification, and obtained the filtrate, whose content as determined by liquid chromatography was 99.58% (calculated by external standard method), and the yield was 90.9%.
[0029] (2) Hydrogenation reduction of methyl 2-hydroxy-4-nitrobenzoate: the above filtrate is placed in an autoclave, 5-10g Pd / C is added, and the nitro group is hydrogenated and reduced at 0.3MPa and 50°C to react to After the end point (sampling for TLC determination), the catalyst was removed by filtration to obtain a filtrate. The content determined by liquid chromatography is 99.60% (calculated by external standard method), and the yield is 92.5%.
[0030] (3) Acetylation of 2-hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com