The preparation method of tolvaptan intermediate
A technology for tolvaptan and intermediates, applied in the field of drug synthesis, can solve problems such as high cost, lack of market competitiveness, and large quantities, and achieve the effects of improving product quality and yield, simple and feasible process methods, and simplified processing methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
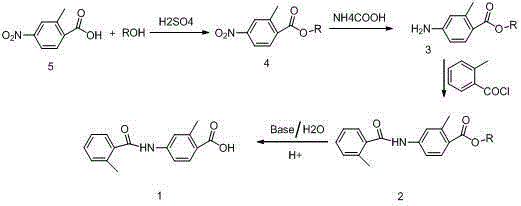
[0037] Embodiment 1: Preparation of methyl 4-nitro-2-methylbenzoate (general formula 4)
[0038] Weigh 18.1g of 4-nitro-2-methylbenzoic acid, add 181g of anhydrous methanol, stir to dissolve, then slowly add 1.81g of concentrated sulfuric acid, heat up to the boiling point of methanol, reflux reaction, TLC (thin layer chromatography) tracking to The raw material (4-nitro-2-methylbenzoic acid) disappeared, cooled to below 30°C, concentrated under negative pressure to recover methanol to dryness, added 181mL ethyl acetate, stirred to dissolve, added 180mL water and stirred to separate layers, and the organic layer solution was re- Wash twice with saturated brine (90mL×2), add an appropriate amount of anhydrous sodium sulfate and dry for 1.5 hours; filter off the desiccant, and seal the filtrate for later use.
Embodiment 2
[0039] Embodiment 2: Preparation of ethyl 4-nitro-2-methylbenzoate (general formula 4)
[0040] Weigh 18.1g of 4-nitro-2-methylbenzoic acid, add 181g of absolute ethanol, stir and dissolve, then slowly add 1.81g of concentrated sulfuric acid, heat up to the boiling point of ethanol, reflux reaction, TLC tracking to the raw material (4-nitrate base-2-methylbenzoic acid) disappeared, lowered the temperature to below 30°C, concentrated under negative pressure to recover ethanol to dryness, added 180mL ethyl acetate, stirred to dissolve, added 180mL water and stirred to separate layers, and the organic layer solution was washed with saturated saline ( 90mL×2) was washed twice, added an appropriate amount of anhydrous sodium sulfate, dried for 1.5 hours, filtered off the desiccant, and the filtrate was sealed for later use.
Embodiment 3
[0041] Embodiment 3: Preparation of methyl 4-amino-2-methylbenzoate (general formula 3)
[0042] In the solution obtained in Example 1, add 1.45g (on a dry basis) of 10% palladium carbon and 108g ammonium formate, stir and react at 45°C until the raw materials disappear, filter off the palladium carbon, wash and filter with a small amount of ethyl acetate Cake once, combine the organic layer solution, wash with saturated brine (60mL×2 times), separate layers, dry over anhydrous sodium sulfate for 1 hour, filter off the desiccant, concentrate under negative pressure to recover ethyl acetate, and obtain light yellow crystal 4 -Methyl amino-2-methylbenzoate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com