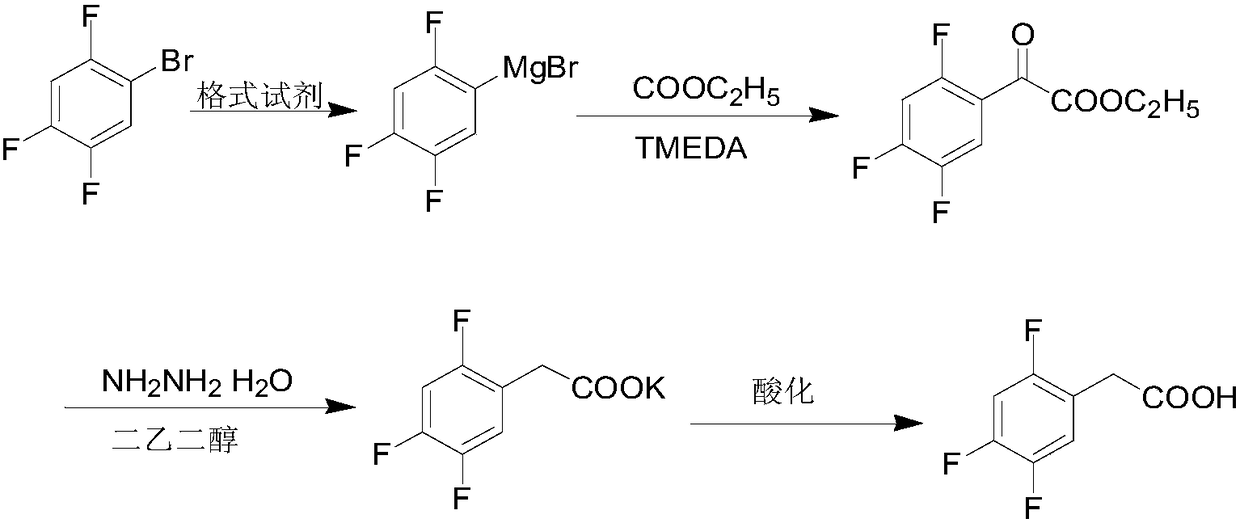
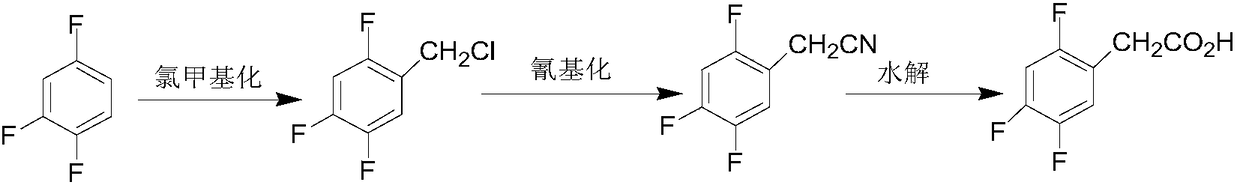
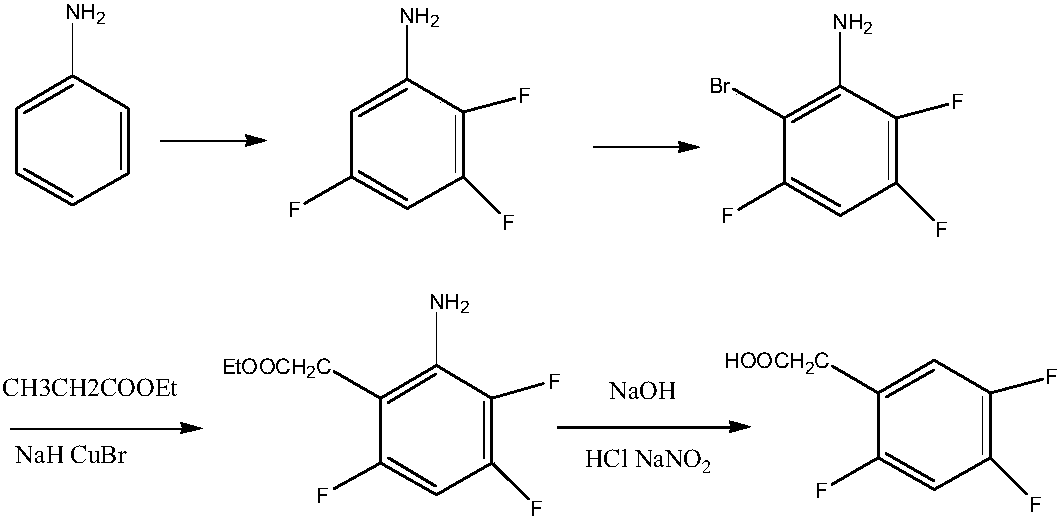
Preparation method of 2,4,5-trifluorophenylacetic acid
A technology of trifluorophenylacetic acid and fluoroborate, which is applied in 2 fields, can solve problems such as poor safety, and achieve the effect of high safety, good comprehensive effect and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Put 36.5g of 2-methyl-4-nitrobenzoic acid and 200mL of glacial acetic acid into the reactor, then slowly cool down to below 5°C and then put in 17g of sodium nitrite, after the end, add 14g of hydrofluoric acid dropwise, drop After completion, control the temperature below 5°C to carry out the fluorination reaction. After the central control confirms that the raw materials have been reacted, then raise the temperature at 20°C to 25°C for pyrolysis for 30 minutes, then add 150mL of dichloromethane and 50mL of water, stir for 30 minutes, and then statically Place and separate layers to obtain a dichloromethane solution containing the compound of formula II, which is directly used in the next reaction, and the single-step yield reaches 95.8%;
[0043] Put the above-mentioned methylene chloride solution containing the compound of formula II into another reaction bottle, then put in 16g of pyridine, control the temperature at room temperature, add 36g of trichloroacetyl chlor...
Embodiment 2
[0046] Put 36.5g of 2-methyl-4-nitrobenzoic acid and 200mL of formic acid into the reactor, then slowly lower the temperature to below 5°C and then put in 20.7g of sodium nitrite. After the completion, control the temperature below 5°C to continue the fluorination reaction. After the central control confirms that the raw materials have been reacted, then heat up at 20°C to 25°C for 30 minutes of pyrolysis, then add 150mL of dichloromethane and 50mL of water, and stir for 30 minutes. After standing and layering, the obtained dichloromethane solution containing the compound of formula II was directly used in the next reaction, and the single-step yield reached 94.6%;
[0047] Put the above dichloromethane solution containing the compound of formula II into another reaction bottle, then put in 18g of pyridine, control the temperature at room temperature, add 40g of trichloroacetyl chloride dropwise, and make the reaction complete after the dropwise addition, then transfer the mate...
Embodiment 3
[0050] Put 36.5g of 2-methyl-4-nitrobenzoic acid and 200mL of formic acid into the reactor, then slowly cool down to below 10°C and then put in 29.5g of sodium nitrite, after the end, add 82g of sodium fluoroborate dropwise, drop After completion, control the temperature at 5°C to 10°C to continue the fluorination reaction. After the central control confirms that the raw materials have been reacted, then heat up at 20°C to 25°C for 30 minutes of pyrolysis, then add 200 mL of ethyl acetate and 50 mL of water, and stir for 30 minutes. After standing still and layering, the obtained ethyl acetate solution containing the compound of formula II was directly used in the next reaction, and the single-step yield reached 94.8%;
[0051] Put the above-mentioned ethyl acetate solution containing the compound of formula II into another reaction bottle, and then put in 30g of diisopropylethylamine, under the condition of controlling the temperature at 20-25°C, add 42g of trichloroacetyl chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com