Application of tribenzothiazolyl benzene to nitryl aroma explosive fluorescence detection
A technology of tribenzothiazolylbenzene and benzothiazolylbenzene, which is applied in the application field of tribenzothiazolylbenzene, can solve problems such as few reports in the literature, achieves good trace detection, low detection limit, and responsiveness short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of Tribenzothiazolylbenzene
[0038] The chemical reaction equation is shown in Reaction Formula II:
[0039]
[0040] Specifically include the following steps:
[0041] Weigh 3mmol of trimesic acid and 9.2mmol of 2-aminothiophenol, add them into the reaction bottle, then add the catalyst polyphosphoric acid (PPA), and react at 150°C for 24 hours. After the reaction was completed, after cooling to room temperature, the pH was adjusted to alkaline with 1 mol / L NaOH solution, and a large amount of solids precipitated after standing still. Suction filtration, take the solid sample to obtain the crude product, recrystallize with dichloromethane to obtain the target compound;
[0042] The structural formula and characterization of the synthesized tribenzothiazolylbenzene are as follows:
[0043]
[0044] White solid, yield 0.4813g, yield 33.6%, m.p.>300℃; 1 H NMR (400MHz, CDCl 3 ,TMS): δ=7.43-7.50(3H,m,ArH-7,7',7"), 7.53-7.60(3H,m,ArH-6,6',6"), 7.99(3H,d...
Embodiment 2
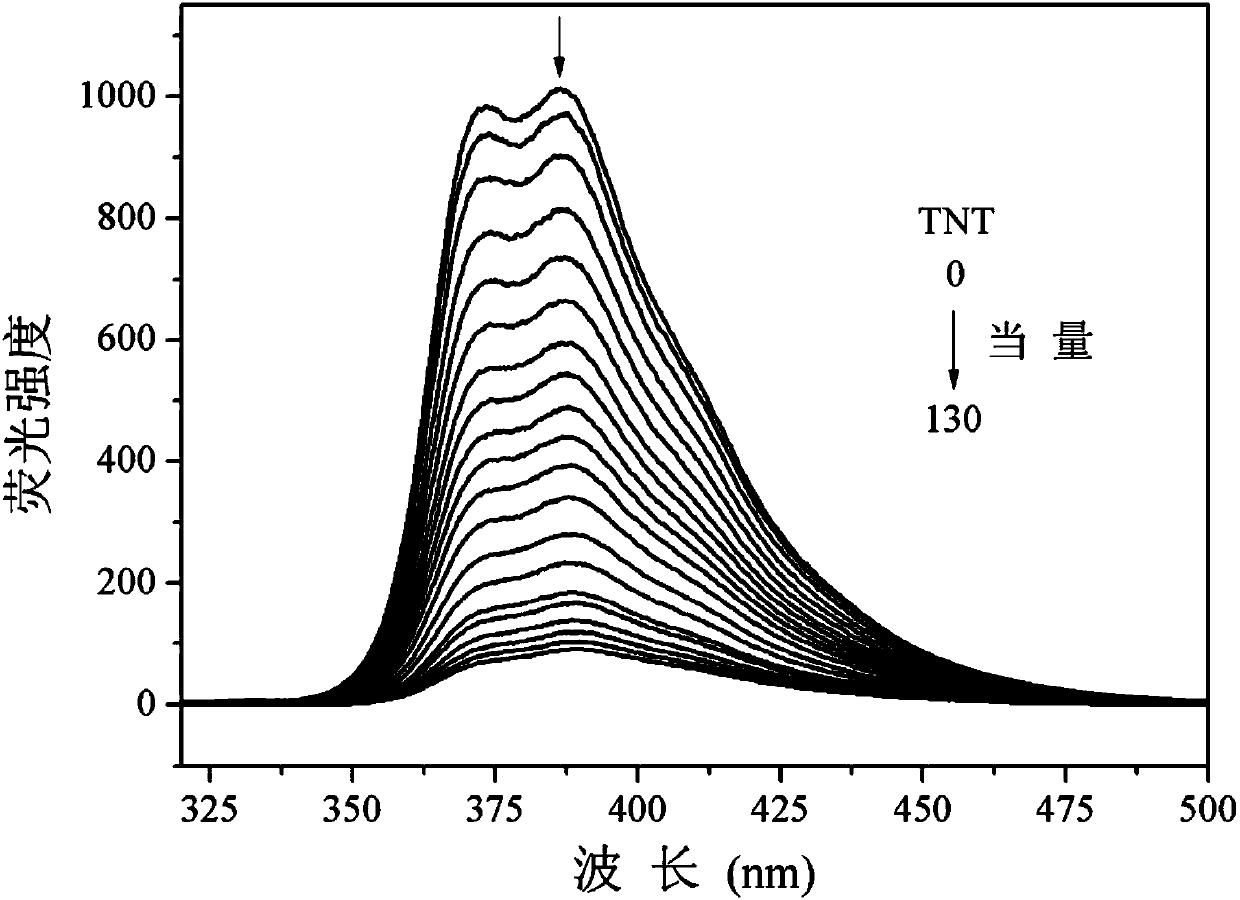
[0046] Fluorescence titration of tribenzothiazolylbenzene to 2,4,6-trinitrotoluene (TNT) prepared in Example 1
[0047] In the tetrahydrofuran-water solution (volume ratio 6:4) of tribenzothiazolylbenzene (10 μM), TNT dissolved in toluene was added dropwise, excited at 300 nm light, and the influence of TNT on the fluorescence properties of tribenzothiazolylbenzene was tested. The result is as figure 1 shown. Depend on figure 1 It can be seen that with the gradual addition of TNT, the fluorescence of tribenzothiazolylbenzene is gradually quenched; when the fluorescence is basically quenched, the amount of TNT to be added is 130 equivalents.
Embodiment 3
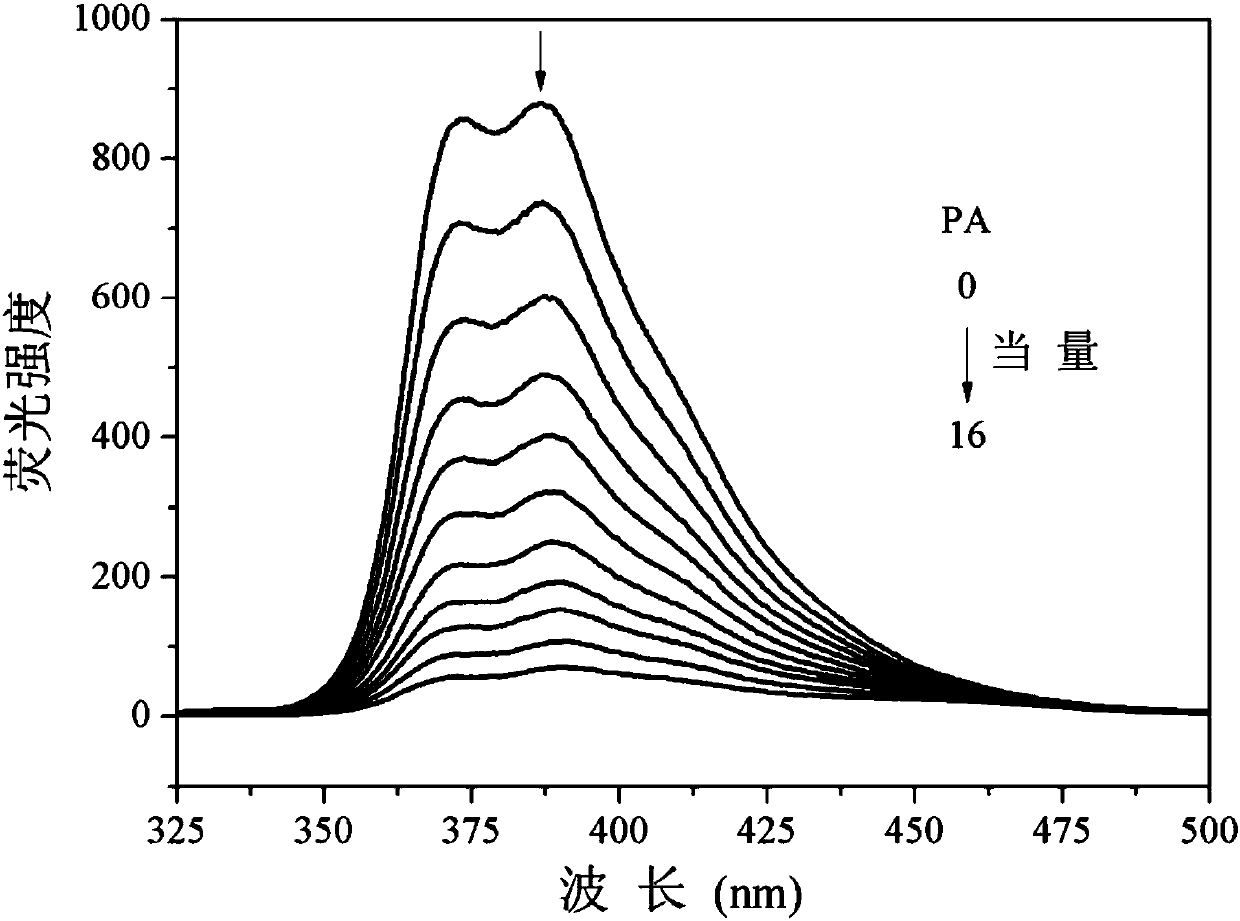
[0049] Fluorescent titration of tribenzothiazolylbenzene to 2,4,6-trinitrophenol (PA) prepared in Example 1
[0050] In the tetrahydrofuran-water solution (volume ratio 6:4) of tribenzothiazolylbenzene (10 μM), PA dissolved in tetrahydrofuran was added dropwise, excited at 300 nm, and the influence of PA on the fluorescence properties of tribenzothiazolylbenzene was tested. The result is as figure 2 shown. Depend on figure 2 It can be seen that with the gradual addition of PA, the fluorescence of tribenzothiazolylbenzene is gradually quenched; when the fluorescence is basically quenched, the amount of PA to be added is 16 equivalents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com